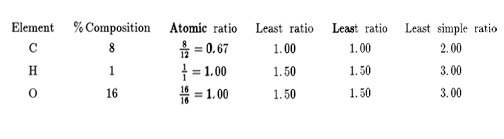A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-SOME BASIC CONCEPTS OF CHEMISTRY-MULTIPLE CHOICE QUESTIONS
- RH2 (ion exchange resin) can replace Ca^(2+) ions in hard water as: ...
Text Solution
|
- 150 mL 0.08 M BaCl2 is added to 100 mL 0.1 M Al(2)(SO(4))(3) and it is...
Text Solution
|
- 1g silver salt of an organic dibasic acid on heating yields 0.5934 g A...
Text Solution
|
- 2 mol N2 and 3 mol H2 are allowed to react in a 20 L flask at 400 K an...
Text Solution
|
- A mixture containing 28 g Cao and 20 g NaOH is treated with aqueous HC...
Text Solution
|
- On warming, H2O2 in aqueous solution decomposes as 2H(2)O(2)(aq) to 2...
Text Solution
|
- A commercial sample of H2O2 is marked as 33.6 V. The molarity of H2O2 ...
Text Solution
|
- 25.4 g of iodine and 14.2 g chlorine react to give a mixture of ICl an...
Text Solution
|
- On subjecting 10 ml mixture of N2 and Co to repeated electric spark to...
Text Solution
|
- 0.70 g sample consisting of CaC2O4 and MgC2O4 is heated at 300 °C to c...
Text Solution
|
- Nitric acid is produced from NH(3) in the following three steps, (I...
Text Solution
|
- The molecular formula of a commercial resin used for exchanging ions i...
Text Solution
|
- In Carius method of estimation of halogens, 250 mg of an organic compo...
Text Solution
|
- In the reaction 4A + 2B + 3C to A4 B2 C3, what will be the number mole...
Text Solution
|
- Study the following table. Which of these two compounds have the ...
Text Solution
|
- For the reaction, CX(4) + 2O(2) to CO(2) + 2X(2)O 0.9 g CX(4) com...
Text Solution
|
- A carbon compound contains 12.8% of carbon, 2.1% of hydrogen and 85.1%...
Text Solution
|
- If 6.3 g of NaHCO3 are added to 15.0 g CH3 COOH solution. The residue ...
Text Solution
|
- How many carbon atoms are present in 0.35 mole of C(6)H(12)O(6)? (Giv...
Text Solution
|
- The vapour density of a mixture containing NO2 and N2O4 is 38.3 at 27°...
Text Solution
|