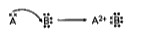A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
EDUCART PUBLICATION-SAMPLE PAPER 5-SECTION B
- Name the oxidising and reducing agent in the following reaction: 2H(...
Text Solution
|
- Equal volumes of hydrochloric acid and potassium hydroxide solutions o...
Text Solution
|
- During the formation of a compound between two atoms A and B. Atom A l...
Text Solution
|
- Which of the metals react with dilute Nitric acid and evolve hydrogen ...
Text Solution
|
- A small amount of a substance X is taken in a beaker and dilute hydroc...
Text Solution
|
- Deeksha performed an experiment using zinc granules and sodium carbona...
Text Solution
|
- Assertion(A): The acids must always be added slowly to water with cons...
Text Solution
|
- Assertion A: When hydrogen and nitrogen combines, ammonia is formed. ...
Text Solution
|
- Select the incorrect statements (I) Almost alll metals combine with o...
Text Solution
|