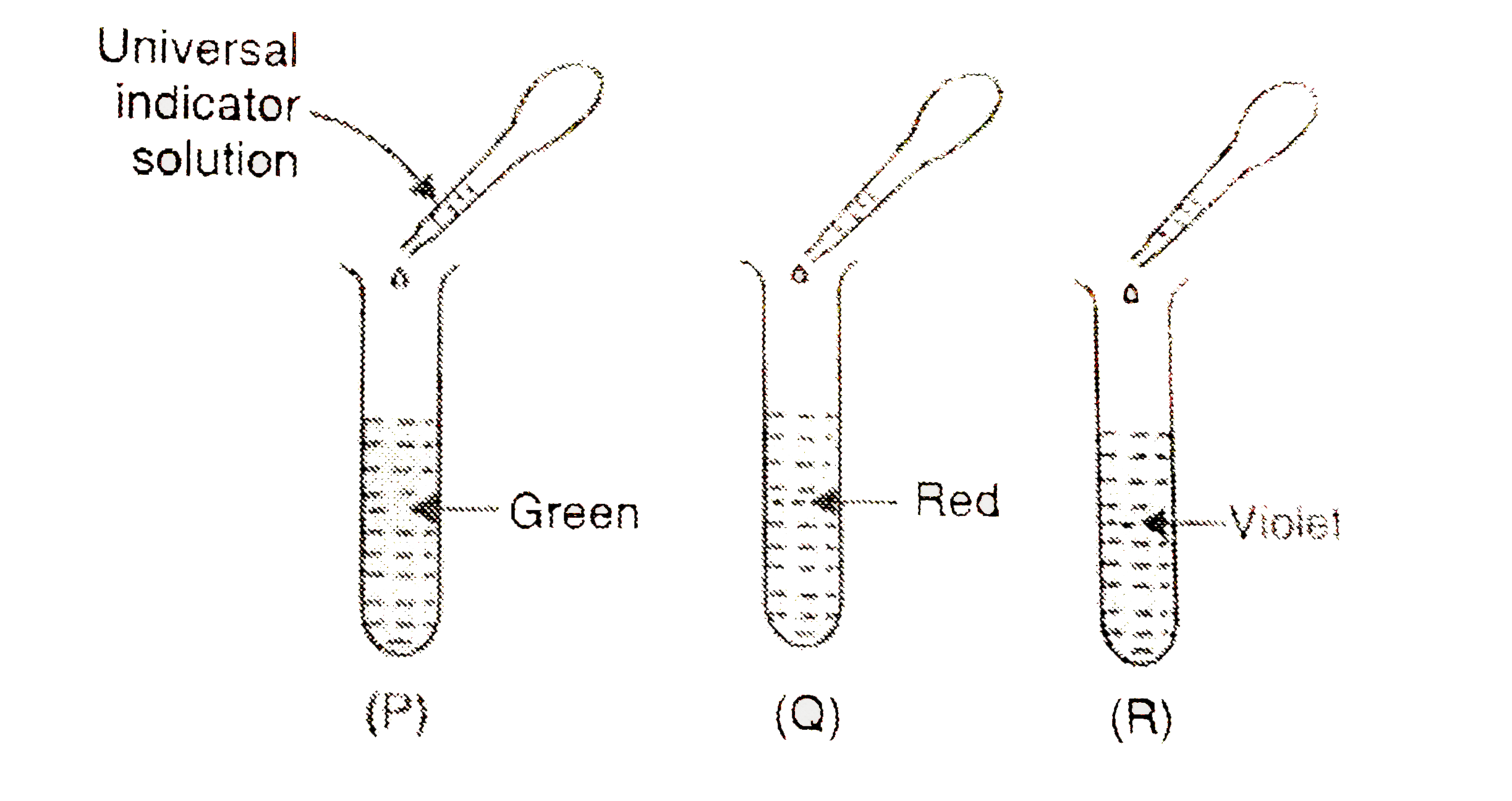A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
EDUCART PUBLICATION-SAMPLE PAPER 8 -SECTION - C
- On adding a few drops of universal indicator solution to three unknown...
Text Solution
|
- Case 1: Prisha is baker. She added the ingredients according to the re...
Text Solution
|
- Case 1: Prisha is baker. She added the ingredients according to the re...
Text Solution
|
- Case 1: Prisha is baker. She added the ingredients according to the re...
Text Solution
|
- Case 1: Prisha is baker. She added the ingredients according to the re...
Text Solution
|
- Case 2: Plants use relatively slow transport system as they have low e...
Text Solution
|
- Case 2: Plants use relatively slow transport system as they have low e...
Text Solution
|
- Case 2: Plants use relatively slow transport system as they have low e...
Text Solution
|