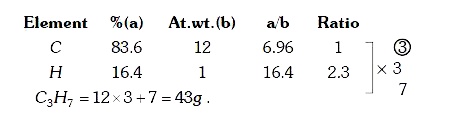A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS( THE MOLE CONCEPT)|38 VideosSOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS( PERCENTAGE COMPOSITION & MOLECULAR FORMULA)|9 VideosSOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS(LAWS OF CHEMICAL COMBINATION )|13 VideosREDOX REACTIONS
ERRORLESS|Exercise ASSERTION & REASON|6 VideosSTATES OF MATTER
ERRORLESS|Exercise ASSERTION AND REASON|11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-SOME BASIC CONCEPTS OF CHEMISTRY-NCERT BASED QUESTIONS(ATOMIC, MOLECULAR AND EQUIVALENT MASSES)
- When 2.46 of hydrated salt (MSO(4)x H(2)O) is completely dehydrated, 1...
Text Solution
|
- In a mole of water vapours at STP, the volume actually occupied or tak...
Text Solution
|
- Complete combustion of 0.858 g of compound X gives 2.63 g of CO(2) and...
Text Solution
|
- What is the mass percent of carbon in carbon dioxide ?
Text Solution
|
- 0.75 g platinic chloride, a monoacid base on ignition gave 0.245 g pla...
Text Solution
|
- Sulphur forms the chlorides S(2)Cl(2) and SCl(2). The equivalent mass ...
Text Solution
|
- Equivalent weight of crystalline oxalic acid is
Text Solution
|
- The equivelent weight of an element is 4. Its chloride has a V.D 59.25...
Text Solution
|
- Assuming fully decomposed, the volume of CO(2) released at STP on heat...
Text Solution
|
- A gaseous mixture contain CH(4) and C(2)H(6) in equimolecular proporti...
Text Solution
|
- One gram of hydrogen is found to combine with 80g of bromine and one g...
Text Solution
|
- In the conversion NH(2)OH rarr N(2)O, the equivalent weight of NH(2)OH...
Text Solution
|
- 0.32 g of metal gave on treatment with an acid 112 mL of hydrogen at N...
Text Solution
|
- To dissolve 0.9 g metal, 100 mL of 1 N HCl is used. What is the equiva...
Text Solution
|
- In the reaction of sodium thiosulphate with I(2) in aqueous medium, th...
Text Solution
|
- What is the volume of water consumed during acid hydrolysis of 1.3...
Text Solution
|
- One litre hard water contains 12.00 mg Mg^(2+) millieqivalent of washi...
Text Solution
|
- 3.92 g of ferrous ammonium sulphate crystals are dissolved in 100 mL o...
Text Solution
|
- In the following reaction, which choice has value twice that of the eq...
Text Solution
|
- M is molecular weight of KMnO(4). The equivalent weight of KMnO(4) whe...
Text Solution
|