A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS( CHEMICAL STOICHIOMETRY AND METHOD OF CONCENTRATION EXPRESSION)|33 VideosSOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS|Exercise PAST YEARS QUESTIONS|24 VideosSOME BASIC CONCEPTS OF CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS( THE MOLE CONCEPT)|38 VideosREDOX REACTIONS
ERRORLESS|Exercise ASSERTION & REASON|6 VideosSTATES OF MATTER
ERRORLESS|Exercise ASSERTION AND REASON|11 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-SOME BASIC CONCEPTS OF CHEMISTRY-NCERT BASED QUESTIONS( PERCENTAGE COMPOSITION & MOLECULAR FORMULA)
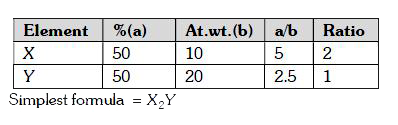
- The simplest formula of a compound containing 50% of element X (atomic...
Text Solution
|
- The empirical formula and molecular mass of a compound are CH(2)O and ...
Text Solution
|
- The empirical formula of an acid is CH(2)O(2), the probable molecular ...
Text Solution
|
- An organic compound whose empirical and molecular formula are same, ca...
Text Solution
|
- The value of 'X' in KAl(SO(4))x.12H(2)O is-
Text Solution
|
- The percentage of nitrogen by mass in ammonium sulphate is closest to ...
Text Solution
|
- 0.1 mol of a carbonhydrate with empirical formula CH(2)O contains 1 g ...
Text Solution
|
- One mole of one of sodium salt listed below, having carbon content clo...
Text Solution
|
- 1.25 g of a metal (M) reacts with oxygen completely to produce 1.68 g ...
Text Solution
|