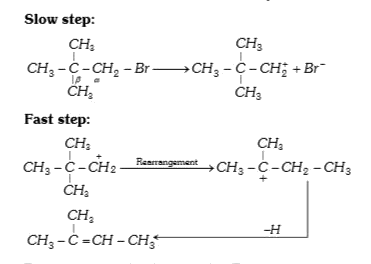
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (Structural and Stereo Isomerism )|99 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise PAST YEARS QUESTIONS |85 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (Dipole Moment, Resonance and Reaction Intermediates )|70 VideosENVIRONMENTAL CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|7 VideosHYDROCARBONS
ERRORLESS|Exercise ASSERTION & REASON|18 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-GENERAL ORGANIC CHEMISTRY -NCERT BASED QUESTIONS (Organic Reactions and their Mechanism )
- Geometry of reaction intermediate in S(N^(1)) reaction is
Text Solution
|
- Which of the following is most reactive toward nucleophilic substituti...
Text Solution
|
- Neopentyl bromide undergoes dehydro halogenation to give alkene even t...
Text Solution
|
- In electrophilic aromatic substitution reaction, the nitro group is me...
Text Solution
|
- Find the product of the given reaction
Text Solution
|
- Match the following Column A to Column B
Text Solution
|
- Dehydrohalogenation in presence of OH^(-) is correctly represented by
Text Solution
|
- The enantiomeric pair among the following four structures
Text Solution
|
- The major product formed in the reaction
Text Solution
|
- Which of the following are correct chain isomers of butane ? (i) <im...
Text Solution
|
- Match the following Column A to Column B
Text Solution
|
- In the reaction benzene with an electrophile E^(+), the structure of t...
Text Solution
|
- The major product in the following reaction is
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- Which of the following are correct chain isomers of butane ? (i) <im...
Text Solution
|
- The order of S(N)1 reactivity in aqueous aceic acid solution for the c...
Text Solution
|
- The species that exhibits the highest R(f) value in a thin layer chrom...
Text Solution
|
- The major product of the following reaction overset(Cu, Delta) (t...
Text Solution
|
- The major product of the reaction between CH(3) CH(2) O Na and (CH(3))...
Text Solution
|
- In the given compounds (A) CH(3)CH(2)CH(2)CH(2)-Br (B) CH(3)-CH(2)...
Text Solution
|