A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise PAST YEARS QUESTIONS |85 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise ASSERTION AND REASON|14 VideosGENERAL ORGANIC CHEMISTRY
ERRORLESS|Exercise NCERT BASED QUESTIONS (Organic Reactions and their Mechanism )|46 VideosENVIRONMENTAL CHEMISTRY
ERRORLESS|Exercise ASSERTION & REASON|7 VideosHYDROCARBONS
ERRORLESS|Exercise ASSERTION & REASON|18 Videos
Similar Questions
Explore conceptually related problems
ERRORLESS-GENERAL ORGANIC CHEMISTRY -NCERT BASED QUESTIONS (Structural and Stereo Isomerism )
- The compound which is not isomeric with diethyl ether is :
Text Solution
|
- Glucose and fructose are
Text Solution
|
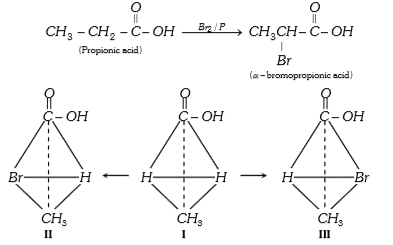
- On bromination, propionic acid gives two isomeric 2-bromopropionic aci...
Text Solution
|
- are
Text Solution
|
- The compound CHCl = CHCHOHCOOH with molecular formuls C(4)H(5)O(3)Cl c...
Text Solution
|
- What type of isomerism is possible for 1-chloro-2-nitroethene?
Text Solution
|
- The correct relation between the following pair of compounds is
Text Solution
|
- Pair is known as
Text Solution
|
- Which of the following compounds can exhibit both geometrical isomeris...
Text Solution
|
- The number of possible isomers for compound C(2) H(3) Cl(2) Br is
Text Solution
|
- The maximum number of isomer for an alkene with the molecular formula ...
Text Solution
|
- What will be total number of isomers of compound with molecular formul...
Text Solution
|
- The total number of acyclic isomers, including the stereoisomers, with...
Text Solution
|
- The number of possible isomers of butene are
Text Solution
|
- The total number of acyclic isomers, including the stereoisomers, with...
Text Solution
|
- A compound is formed by substitution of two chlorine for two hydrogens...
Text Solution
|
- Choose the correct statement(s) among the following.
Text Solution
|
- Indentify the stereoisomer pair from the following choice -
Text Solution
|
- Two possible stereoisomers for are
Text Solution
|
- The following two compounds are
Text Solution
|