A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ERRORLESS-GENERAL ORGANIC CHEMISTRY -ASSERTION AND REASON
- Assertion : Aniline is better nucleophile than aniline ion. Reason :...
Text Solution
|
- Assertion: Neopentane forms one mono substitutes compound Reason: Ne...
Text Solution
|
- Assertion : trans -2-Butene on reaction with Br(2) gives meso-2,3-dibr...
Text Solution
|
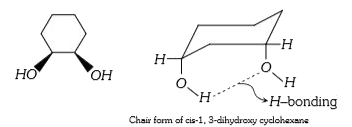
- Assertion : Cis-1,3 dihydroxy cyclohexane exists in chair conformation...
Text Solution
|
- Assertion: Benzyl bromide when kept in acetone water produces benzyl a...
Text Solution
|
- Assetion: Carbon possesses property of catenation. Reason: Carbon at...
Text Solution
|
- (A) Tertiary carbocations are generally formed more easily than primar...
Text Solution
|
- (A) Heterolytic fission involves the breaking of a covalent bond in s...
Text Solution
|
- Assertion : Free radicals are short lived and highly reactive. Reaso...
Text Solution
|
- Assertion : Boiling points of cis-isomers are higher than trans - isom...
Text Solution
|
- Assertion: Diastereoisomers have different physical properties. Reas...
Text Solution
|
- Assertion : The presence of nitro group facilitates nucleophilic subst...
Text Solution
|
- Statement-1: All the hydrogen atoms in CH(2)=C=CH(2) lie in one plane...
Text Solution
|
- Assertion: Vinyl halides do not undergo substitution reaction. Reaso...
Text Solution
|