Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
AAKASH INSTITUTE|Exercise SECTION-A|45 VideosTHERMODYNAMICS
AAKASH INSTITUTE|Exercise SECTION-B|20 VideosTHERMODYNAMICS
AAKASH INSTITUTE|Exercise ASSIGNMENT (Section -D) Assertion-Reason Type Questions|15 VideosTHE SOLID STATE
AAKASH INSTITUTE|Exercise Assignment (SECTION - D) (ASSERTION-REASON TYPE QUESTION)|20 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-THERMODYNAMICS-TRY YOURSELF
- In the equationn pV = nRT, how many state varibles are present?
Text Solution
|
- Temperature is a state functionn or not?
Text Solution
|
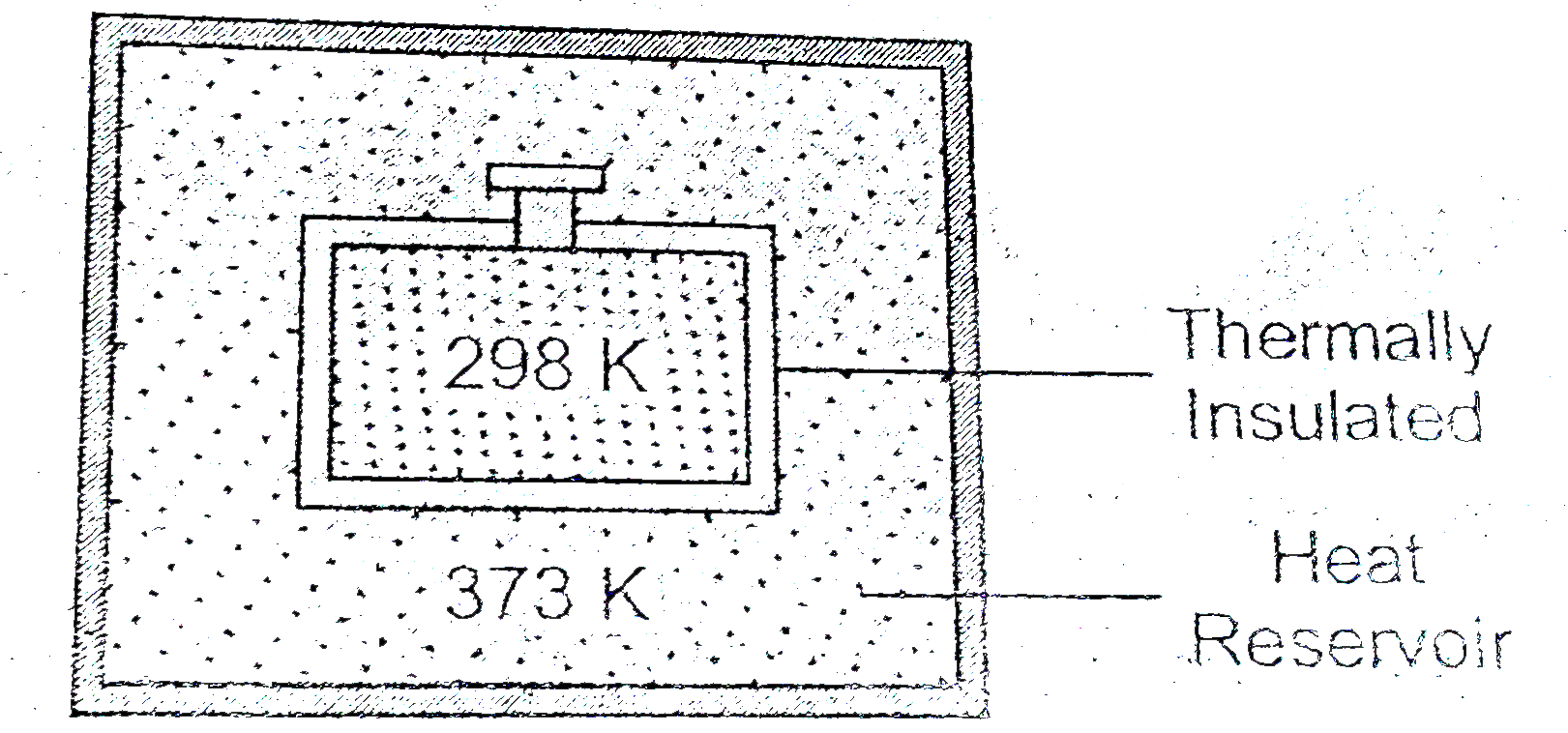
- Consider the following figure andtell the nature of work done.
Text Solution
|
- A beaker containing water at 373 K is representig an open system, is p...
Text Solution
|
- 160 J of work is done on the system and at the same time 100 J of heat...
Text Solution
|
- If change in internal energy -80 J. The work done by system is +40 J. ...
Text Solution
|
- If the external pressure is more than internal pressure then what will...
Text Solution
|
- What would be the sign convention for work done on a system for a cyli...
Text Solution
|
- One mole of oxygen is allowed to expand isothermally and reversibly fr...
Text Solution
|
- How much heat would be absorbed by two litres of an ideal gas at 5 atm...
Text Solution
|
- The heat of combustion of napthalene {C(10)H(8)(s)} at constant volume...
Text Solution
|
- How it is possible to find the value of Delta U and not the value of U...
Text Solution
|
- Out of pressure, mass, moles and volume, which is an intensive propert...
Text Solution
|
- Pick the odd one out : (Intensive or extensive). Refractive index, d...
Text Solution
|
- Calculate the amount of heat required to raise the temperature of 5 g ...
Text Solution
|
- Calculate the weight of gold having specific heat capacity 0.13 J (.^(...
Text Solution
|
- 90 g of water spilled out from a vessel in the room on the floor. Assu...
Text Solution
|
- One mole of carbon undergoes incomplete combustion to produce carbon m...
Text Solution
|
- The standard heat of formation of at 298 K for CCl(4)(g), H(2)O(g), CO...
Text Solution
|
- Why Delta H(f)^(@) of elements is zero?
Text Solution
|