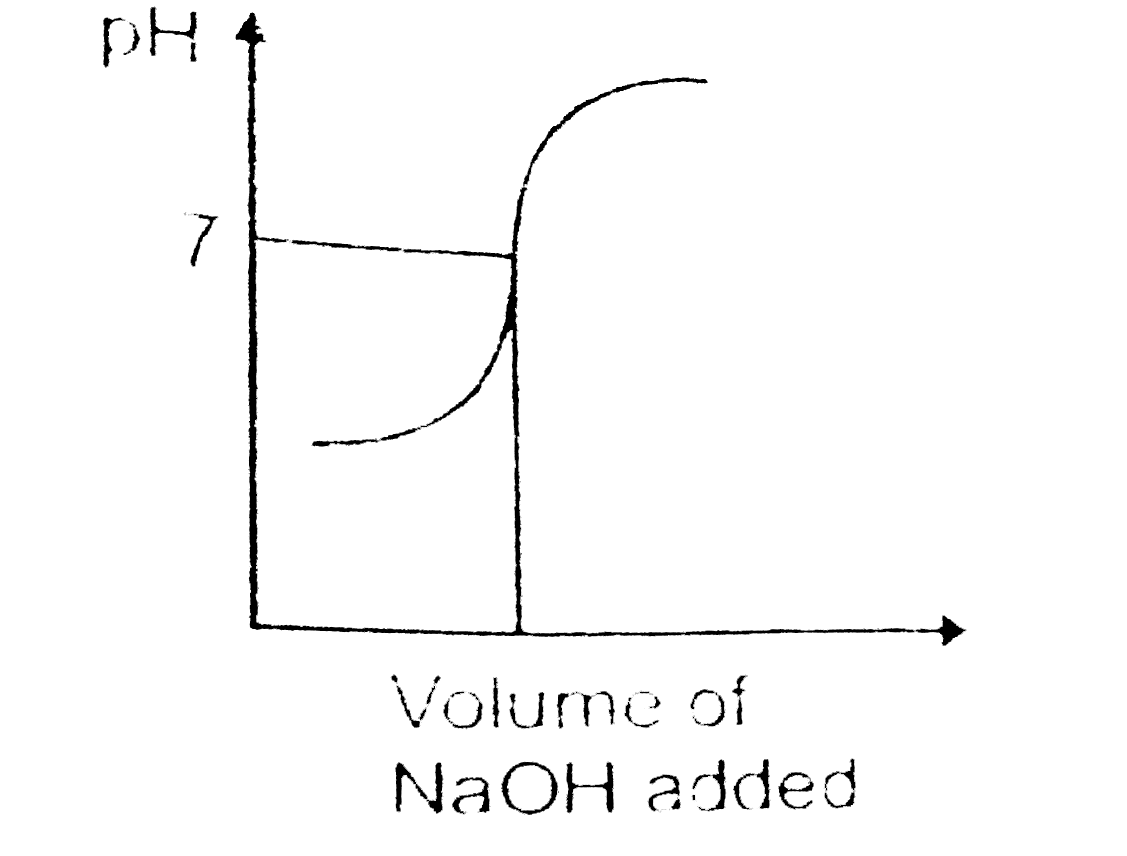
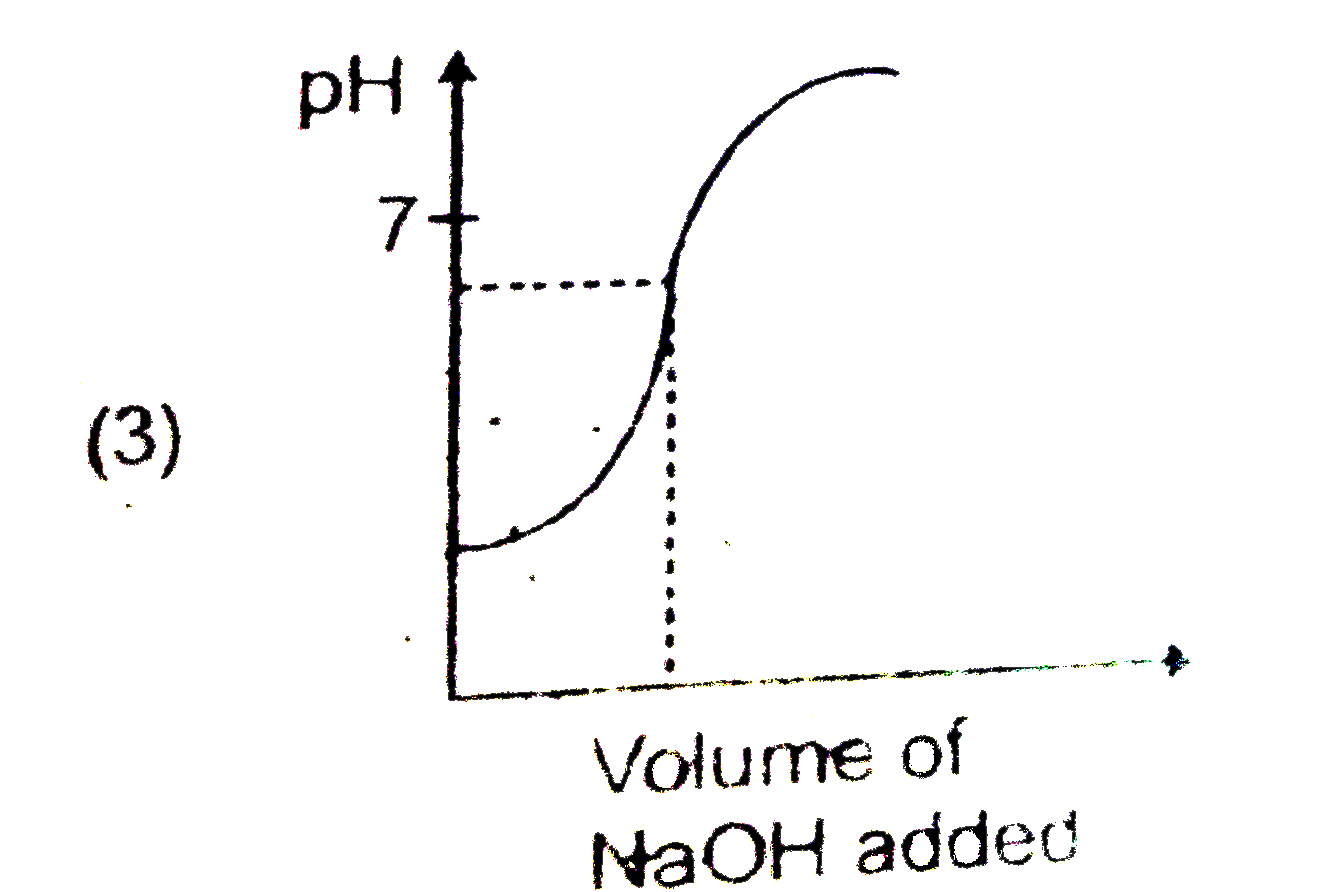
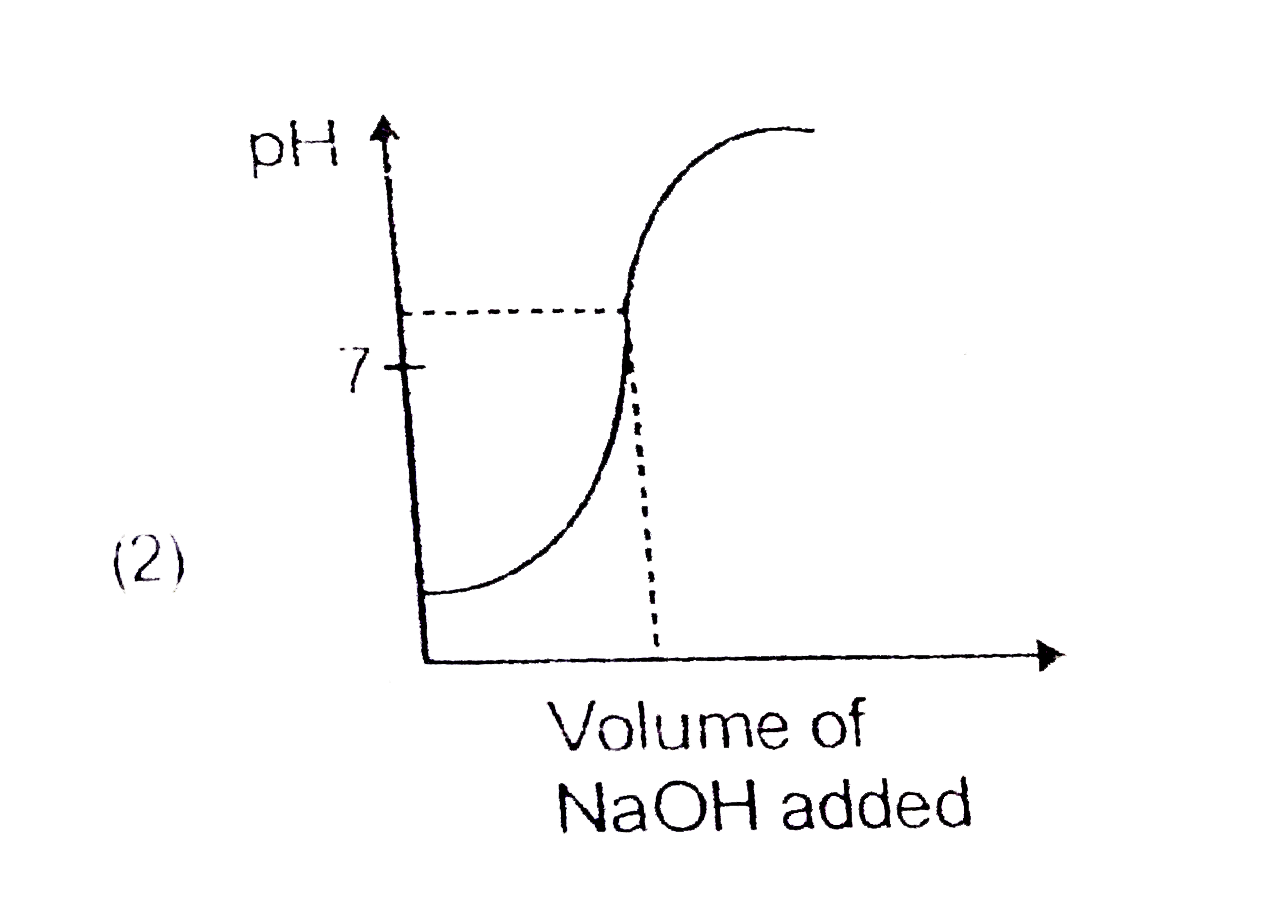A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUILIBRIUM
AAKASH INSTITUTE|Exercise EXERCISE|50 VideosEQUILIBRIUM
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION -A)|50 VideosEQUILIBRIUM
AAKASH INSTITUTE|Exercise Assignment (SECTION-I) (SUBJECTIVE TYPE QUESTIONS)|9 VideosENVIRONMENTAL CHEMISTRY
AAKASH INSTITUTE|Exercise ASSIGNMENT (SECTION-D) (Assertion - Reason Type Questions)|5 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
AAKASH INSTITUTE|Exercise Assignment (Section-D) Assertion-Reason Type Question|15 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-EQUILIBRIUM-Assignment (SECTION-J) (AAKASH CHALLENGERS QUESTIONS)
- The exact concentration of H^(+) ion in 10^(-3) molar HCl aq solution ...
Text Solution
|
- In ammonia formation process, due t o increase in pressure, equlibrium...
Text Solution
|
- 4 mole of N(2)O(4) is taken in container of unit volume at any tempera...
Text Solution
|
- Which of the following pH curve represent the titration of weak acid a...
Text Solution
|
- Which of the followig equilibrium will shift in forward direction on ...
Text Solution
|
- The equilibrium constant of given reaciton will be HCO(3)^(-)+H(2)OhAr...
Text Solution
|