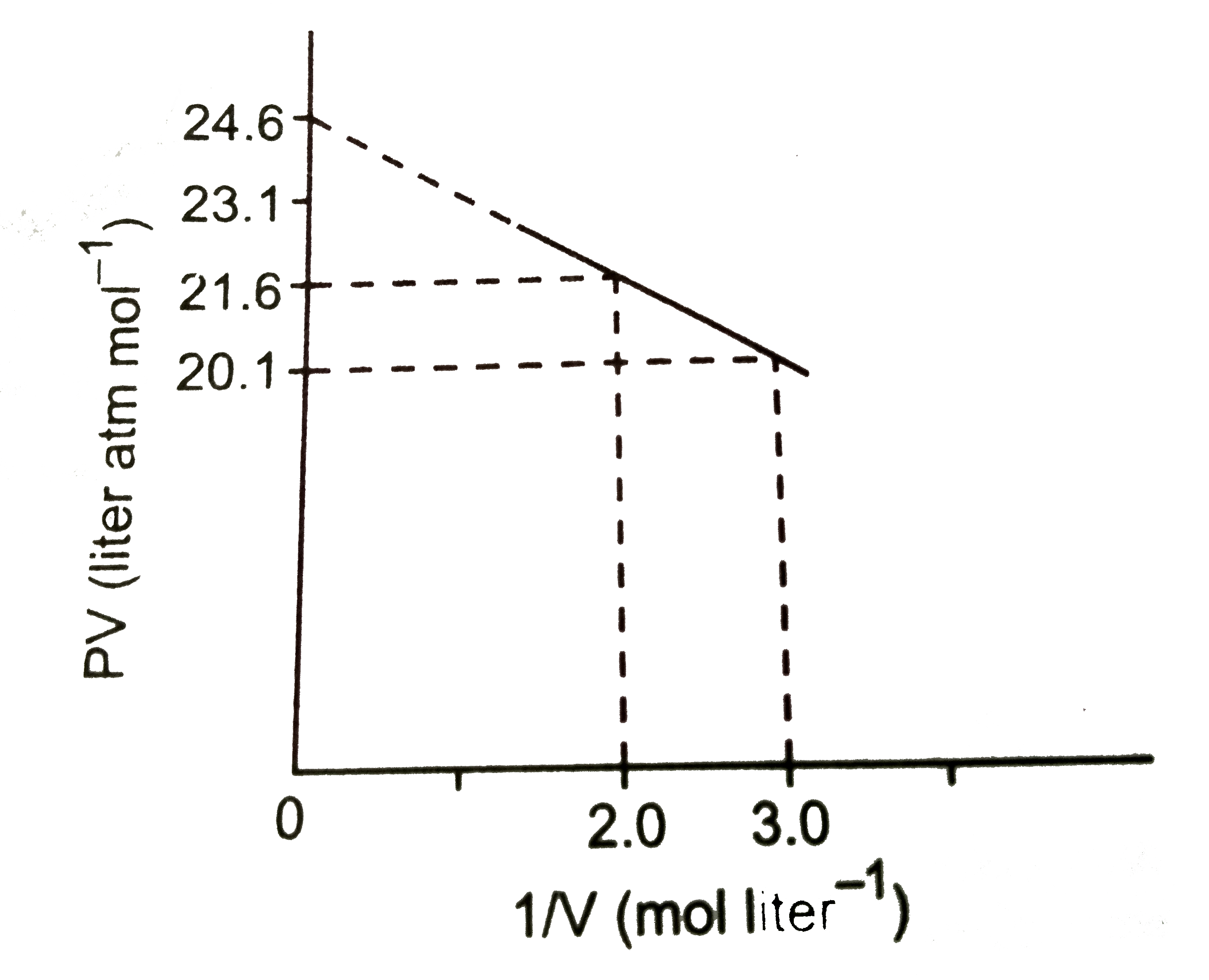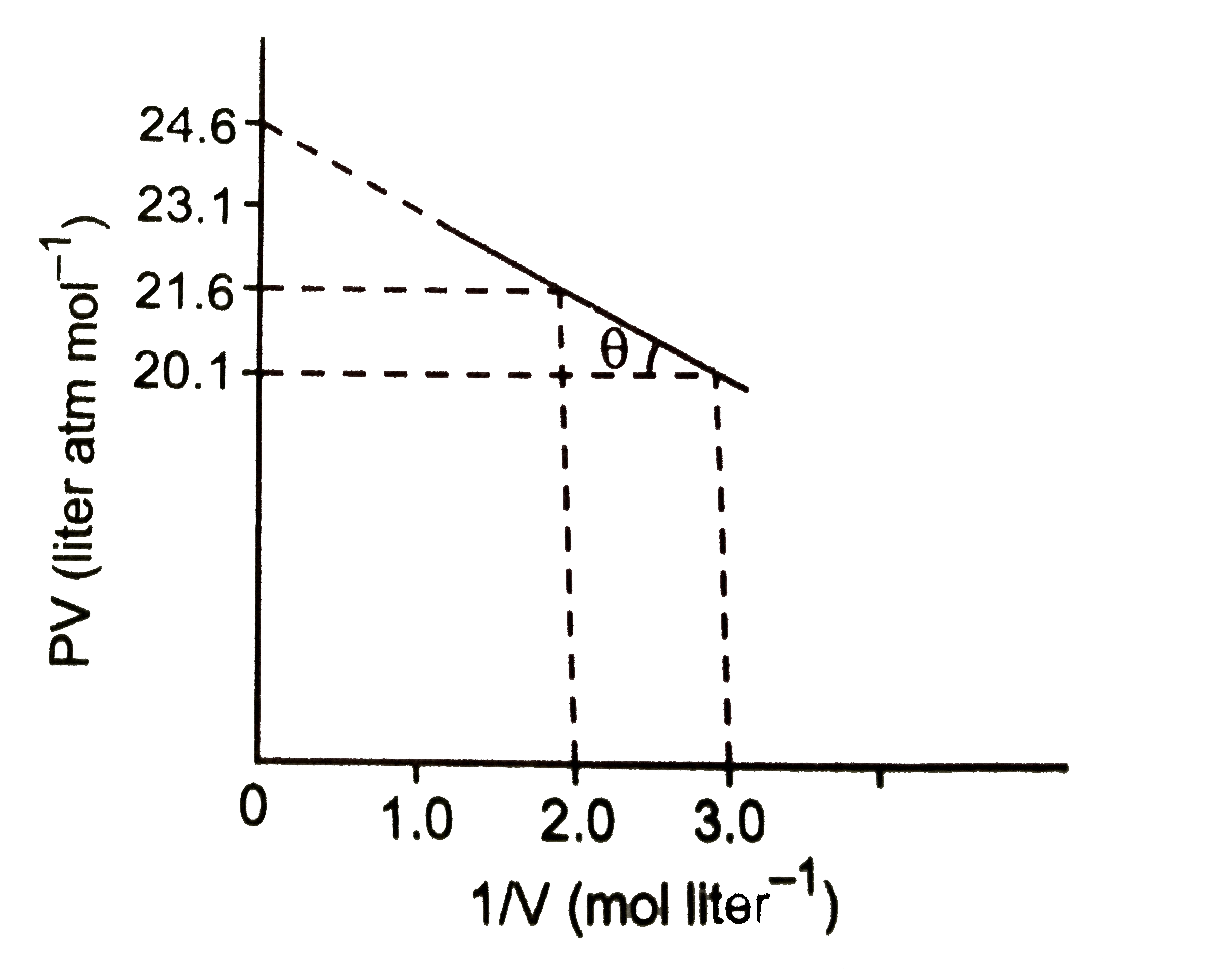A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise COMPETITION FOCUS (Liquefaction of gases and critical temperature)|3 VideosSTATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise COMPETITION FOCUS (Liquid state and properties of liquid)|4 VideosSTATES OF MATTER : GASES AND LIQUIDES
PRADEEP|Exercise COMPETITION FOCUS (Kinetic theory of gases, kinetic energy and molecular speeds|12 VideosSOME p-BLOCK ELEMENTS
PRADEEP|Exercise Competition Focus (JEE( Main and Advanced)/Medical Entrance) VIII. Assertion-Reason Type Questions (Type I)|23 VideosSTATES OF MATTER: SOLID MATTER
PRADEEP|Exercise COMPETITION FOCUS (ASSERTION-REASON)|17 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-STATES OF MATTER : GASES AND LIQUIDES-COMPETITION FOCUS (Behaviour of real gases and van der Waals equation)
- A gas such as carbon monoxide would be most likely to obey the ideal g...
Text Solution
|
- For one mole of a van der Waals gas when b=0 and T=300 K, the PV vs 1/...
Text Solution
|
- What is the pressure of 2 mole of NH(3) at 27^(@)C when its volume is ...
Text Solution
|
- The isotherm obtained for CO is as follows : The compressibility ...
Text Solution
|
- For real gases, van der Waals' equation is written as (P+(an^(2))/(V...
Text Solution
|
- The given graph represents the variation of Z (compressibility factor=...
Text Solution
|
- Under critical conditions, the compressibility factor for a gas is .
Text Solution
|
- a' and 'b' are van der Waals' constants for gases Chlorine is more eas...
Text Solution
|
- Maximum deviation from ideal gas is expected from
Text Solution
|
- If Z is a compressibility factor, van der Waals' equation at low press...
Text Solution
|
- One mole of a monoatomic real gas satisfies the equation p(V-b)=RT wh...
Text Solution
|
- The correction factor 'a' to the ideal gas equation corresponds to
Text Solution
|