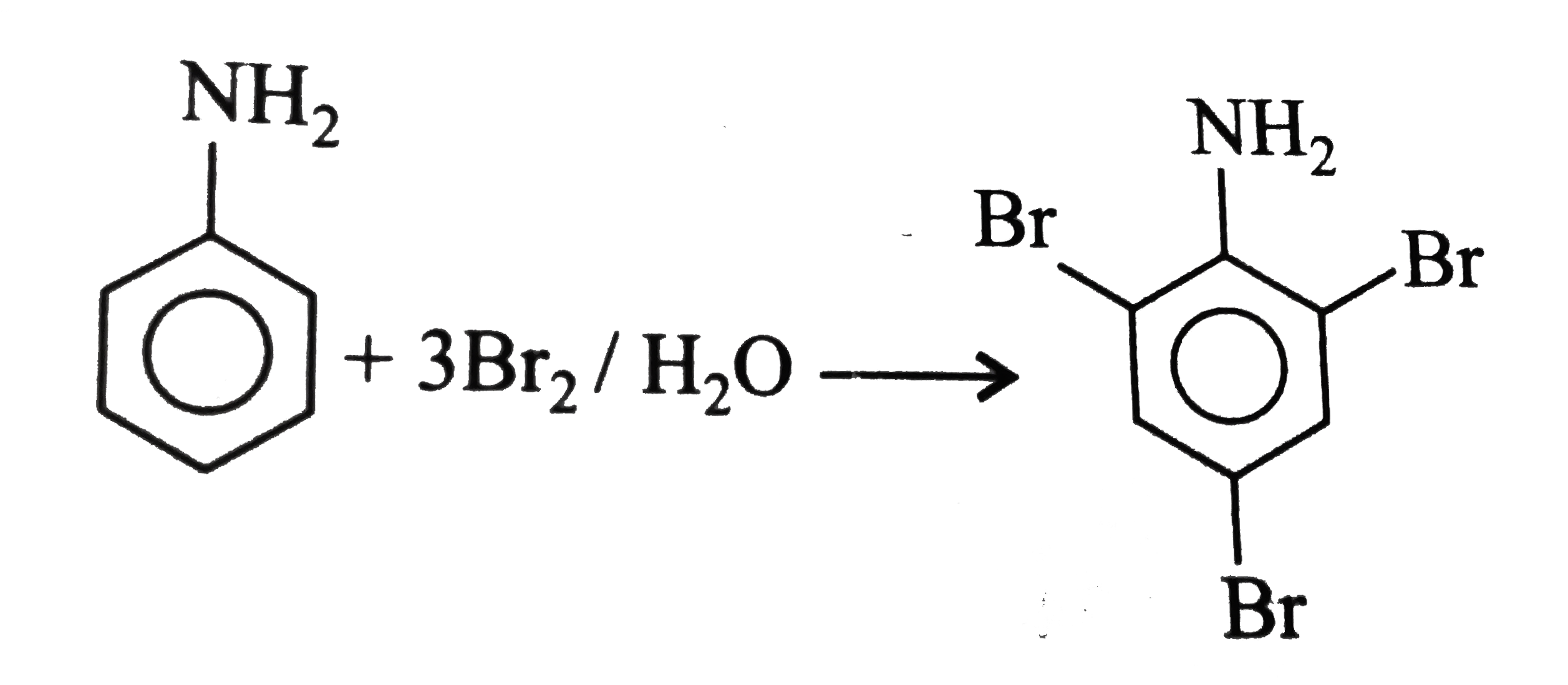
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-STOICHIOMETRY-Archives Subjective
- A sample of pure aniline was dissolved in HCl and diluted to 100 mL wi...
Text Solution
|
- In the analysis of 0.1 of sample of feldspar, 0.118 g of mixture of Na...
Text Solution
|
- Five millilitires of a gas (A) containing only C and H was mixed with ...
Text Solution
|
- (a). Take 1 L of a mixture of CO and CO(2) Pass this mixture through a...
Text Solution
|
- Find out the equivalent weight of H(3) PO(4)in the reaction: Ca(OH)(...
Text Solution
|
- Hydroxylamine reduces Fe^(3+) accoeding to the following reaction: 2...
Text Solution
|
- An organic compound (C(x)H(2y)O(y)) was burnt with twice the amount of...
Text Solution
|
- 4.08 g of a mixture of BaO and an unknown carbonate MCO(3) was heated ...
Text Solution
|
- When 16.8 g of a white solid X was heated 4.4 g of an acid gas A which...
Text Solution
|
- 2.68xx10^(-3) moles of solution containing anion A^(n+) require 1.61xx...
Text Solution
|
- 5 mL of 8 N HNO(3), 4.8 mL of 5N HCl and a certain volume of 17 M H(2)...
Text Solution
|
- What is the weight of sodium bromate and molarity of solution to prepa...
Text Solution
|
- A sample of hydrazine sulphate (N(2)H(6)SO(4)) was dissolved in 100 mL...
Text Solution
|
- An equal volume of reducing agent is titrated separately with 1 M KMnO...
Text Solution
|
- n-butane is produced by the monobromination of ethane followed by Wurt...
Text Solution
|
- A mixture of H(2)C(2)O(4) and NaHC(2)O(4) weighing 2.02 g was dissolve...
Text Solution
|
- A solid mixture 5 g consists of lead nitrate and sodium nitrate was he...
Text Solution
|
- A solution of 0.2 g of a compound containing Cu^(2+) and C(2)O(4)^(2-)...
Text Solution
|
- A 1 g sample of Fe(2)O(3) solid of 55.2% purity is dissolved in acid a...
Text Solution
|
- A 2.0 g sample of a mixture containing sodium carbonate, sodium bicarb...
Text Solution
|
- 1 g sample of AgNO(3) is dissolved in 50 mL of water, It is titrated w...
Text Solution
|