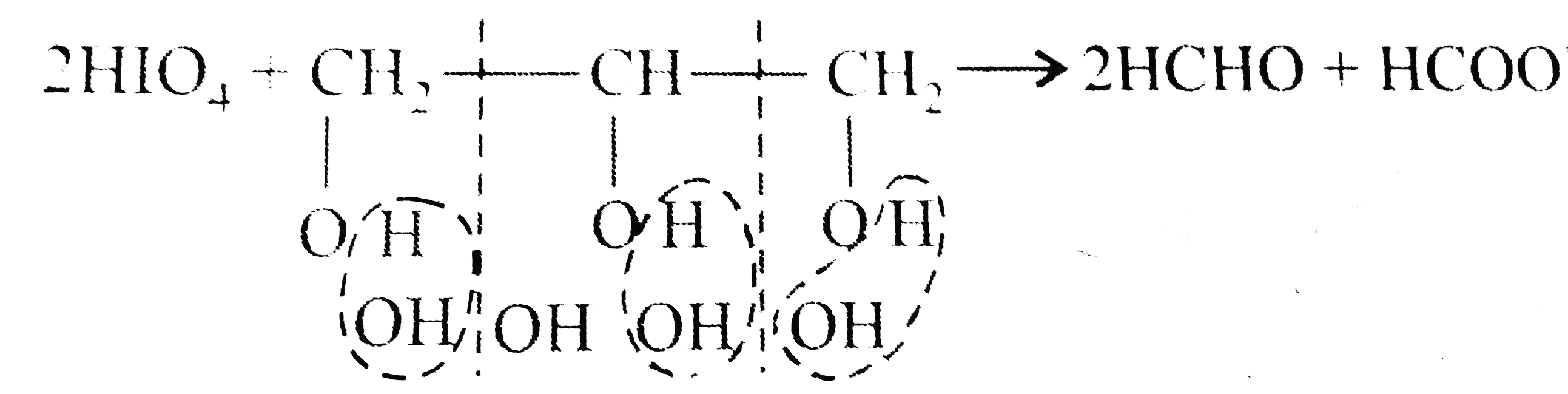A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STOICHIOMETRY
CENGAGE CHEMISTRY|Exercise Exercises Multiple Correct|30 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY|Exercise Exercises Single Correct|85 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY|Exercise Exercises Subjective|31 VideosSTATES OF MATTER
CENGAGE CHEMISTRY|Exercise Exercises (Ture False)|25 VideosTHERMODYNAMICS
CENGAGE CHEMISTRY|Exercise Archives (Subjective)|23 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-STOICHIOMETRY-Exercises Linked Comprehension
- 20 " mL of " (M)/(60) KBrO3 was reacted with a sample of SeO(3)^(2-) 2...
Text Solution
|
- 20 " mL of " (M)/(60) KBrO3 was reacted with a sample of SeO(3)^(2-) 2...
Text Solution
|
- A mixture of CO and CO(2) when treated with I(2)O(5) gives I(2) vapour...
Text Solution
|
- A mixture of CO and CO(2) when treated with I(2)O(5) gives I(2) vapour...
Text Solution
|
- A mixture of CO and CO(2) when treated with I(2)O(5) gives I(2) vapour...
Text Solution
|
- if 20 mL (M)/(10)Ba(MnO(4))(2) compeletely reacts with FeC(2)O(4) in a...
Text Solution
|
- if 20 mL (M)/(10)Ba(MnO(4))(2) compeletely reacts with FeC(2)O(4) in a...
Text Solution
|
- if 20 mL (M)/(10)Ba(MnO(4))(2) compeletely reacts with FeC(2)O(4) in a...
Text Solution
|
- Direct titration of I(2) with a reducing agent is called iodimetry. If...
Text Solution
|
- 638.0 g of CuSO(4) solution is titrated with excess of 0.2 M KI soluti...
Text Solution
|
- Direct titration of I(2) with a reducing agent is called iodimetry. If...
Text Solution
|
- 12 g of impure cyanogen undergoes hydrolysis by two different paths. ...
Text Solution
|
- 12 g of impure cyanogen undergoes hydrolysis by two different paths. ...
Text Solution
|
- 12 g of impure cyanogen undergoes hydrolysis by two different paths. ...
Text Solution
|
- 12 g of impure cyanogen undergoes hydrolysis by two different paths. ...
Text Solution
|
- In the study of titration of NaOH and Na(2)CO(3). NaOH and NaHCO(3), N...
Text Solution
|
- In the study of titration of NaOH and Na(2)CO(3). NaOH and NaHCO(3), N...
Text Solution
|
- H2O2 is reduced rapidly by Sn^(2+). H2O2 is decomposed slowly at room ...
Text Solution
|
- H2O2 is reduced rapidly by Sn^(2+). H2O2 is decomposed slowly at room ...
Text Solution
|
- H2O2 is reduced rapidly by Sn^(2+). H2O2 is decomposed slowly at room ...
Text Solution
|