A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
CENGAGE CHEMISTRY|Exercise Exercises (Archives ) Assertion-Reasoning|1 VideosSOLID STATE
CENGAGE CHEMISTRY|Exercise Exercises (Archives ) Interger|1 VideosSOLID STATE
CENGAGE CHEMISTRY|Exercise Exercises (Archives ) Multiple Correct|2 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY|Exercise SUBJECTIVE TYPE|3 VideosSOLUTIONS
CENGAGE CHEMISTRY|Exercise Ex 2.3 (Objective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SOLID STATE-Exercises (Archives ) Single Correct
- CsBr has bcc structure with edge length of 43 pm. The shortest interio...
Text Solution
|
- The coordination number of a metal crystallizing in a hexagonal close-...
Text Solution
|
- In a solid AB having NaCl structure 'A' atoms occupy the corners & fac...
Text Solution
|
- A substance A(x)B(y) crystallizes in a face-centred cubic lattice in w...
Text Solution
|
- In which of the following crystals, alternate tetrahedral voids are oc...
Text Solution
|
- The packing efficiency of a two-dimensional square unit cell shown bel...
Text Solution
|
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- Experimentally it was found that a metal oxide has formula M(0.98), Me...
Text Solution
|
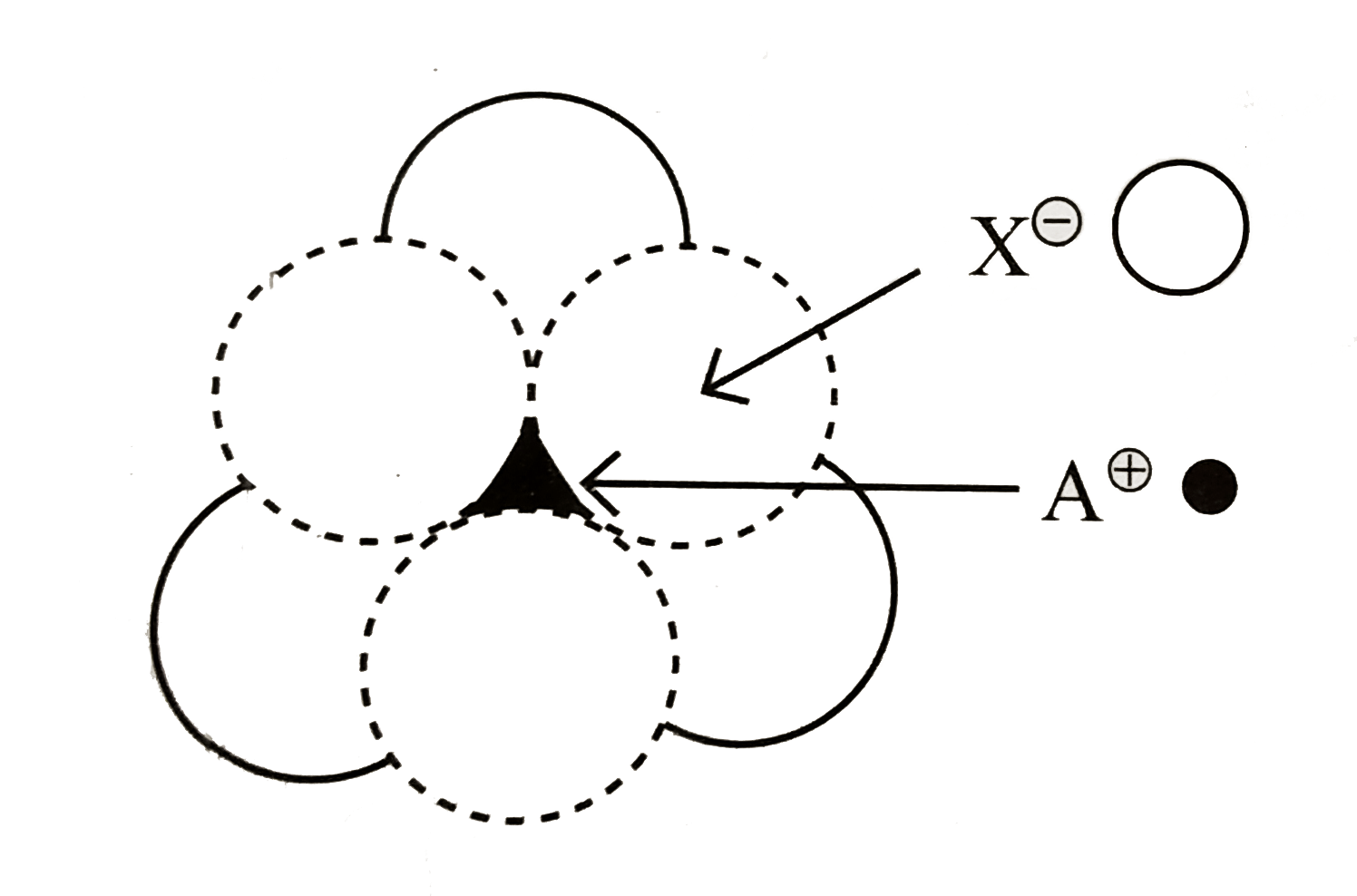
- The arrangement of X^(ɵ) ions around A^(o+) ion in solid AX is given i...
Text Solution
|
- CsClcrystallizes in body centred cubic lattice. If 'a' us its edge len...
Text Solution
|