A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct)|25 VideosSOLUTIONS
CENGAGE CHEMISTRY|Exercise Exercises (Single Correct)|99 VideosSOLUTIONS
CENGAGE CHEMISTRY|Exercise Solved Examples|40 VideosSOLID STATE
CENGAGE CHEMISTRY|Exercise Ex 1.2 (Objective)|9 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY|Exercise Archives Subjective|2 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SOLUTIONS-Exercises (Linked Comprehension)
- The electrolyte solutions show abnormal colligative porperties.To acco...
Text Solution
|
- The electrolyte solutions show abnormal colligative porperties.To acco...
Text Solution
|
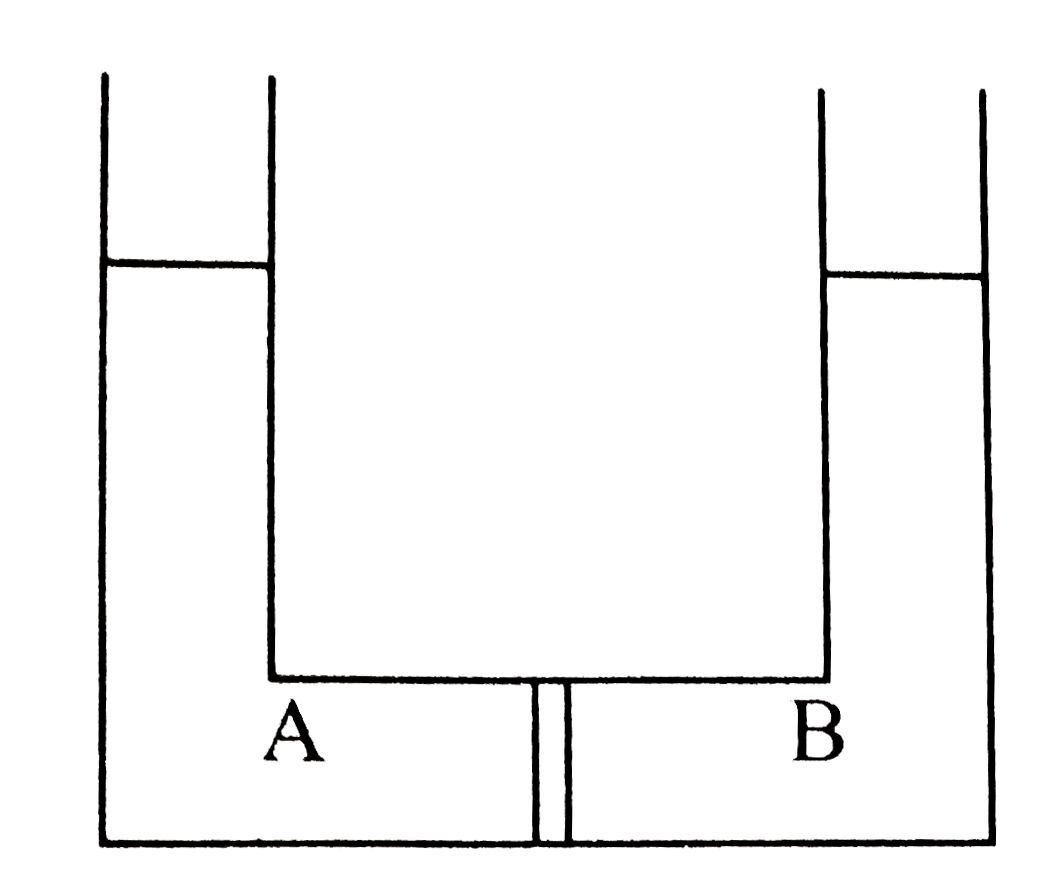
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- Compartment A and B have the following combinations of solution: {:(...
Text Solution
|
- The boiling point elevation and freezing point depression of solution...
Text Solution
|
- The boiling point elevation and freezing point depression of solution...
Text Solution
|
- The boiling point elevation and freezing point depression of solution...
Text Solution
|
- The boiling point elevation and freezing point depression of solution...
Text Solution
|
- The boiling point elevation and freezing point depression of solution...
Text Solution
|
- A solution M is prepared by mixing ethanol and water. The mole fractio...
Text Solution
|
- A solution M is prepared by mixing ethanol and water. The mole fractio...
Text Solution
|
- A solution M is prepared by mixing ethanol and water. The mole fractio...
Text Solution
|
- Properties such as boiling point, freezing point, and vapour pressure ...
Text Solution
|
- Properties such as boiling point, freezing point, and vapour pressure ...
Text Solution
|
- Properties such as boiling point, freezing point, and vapour pressure ...
Text Solution
|
- Properties such as boiling point, freezing point, and vapour pressure ...
Text Solution
|
- A certain vessel X has water and nitrogen gas at a total pressure of 2...
Text Solution
|