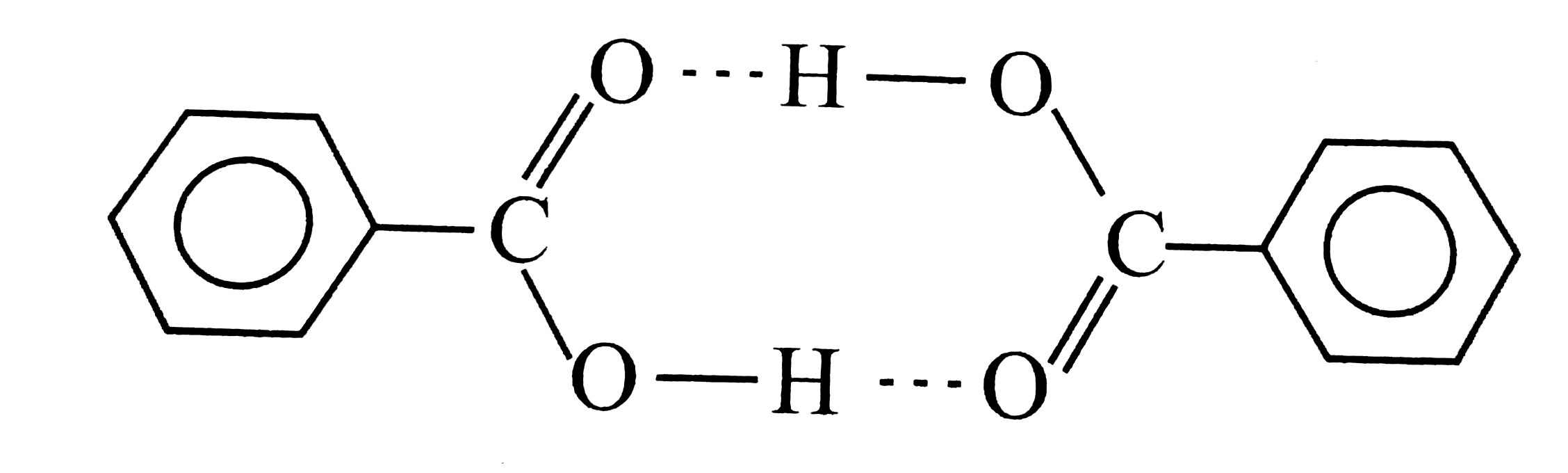A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
CENGAGE CHEMISTRY|Exercise Exercises Archives (Integer)|1 VideosSOLUTIONS
CENGAGE CHEMISTRY|Exercise Exercises Archives (Fill In The Blanks)|1 VideosSOLUTIONS
CENGAGE CHEMISTRY|Exercise Exercises Archives (Linked Comprehension)|3 VideosSOLID STATE
CENGAGE CHEMISTRY|Exercise Ex 1.2 (Objective)|9 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY|Exercise Archives Subjective|2 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SOLUTIONS-Exercises Archives (Multiple Correct)
- In the depression of freezing point experiment, it is found that the:
Text Solution
|
- Benzene and naphthalene from an ideal solution at room temperature. Fo...
Text Solution
|
- An azeotropic solution of two liquid has boiling point lower than eith...
Text Solution
|
- For a dilute solution, Raoult's law states that
Text Solution
|
- A molal solution is one that contains 1 mol of a solute in
Text Solution
|
- When mercuric iodide is added to the aqueous solution of potassium iod...
Text Solution
|
- Which of the following 0.1 M aqueous solutions will have the lowest fr...
Text Solution
|
- The freezing point among the following equimolal aqueous solutions wil...
Text Solution
|
- When 0.004 M Na(2)SO(4) is an isotonic acid with 0.01 M glucose, the d...
Text Solution
|
- The molecular weight of benzoic acid in benzene as determined by depre...
Text Solution
|
- During depression of freezing point in a solution, the following are i...
Text Solution
|
- The elevation in boiling point of a solution of 13.44g of CuCl(2)(mole...
Text Solution
|
- When 20 g of naphthoic acid (C(11)H(8)O(2)) is dissolved in 50 g of be...
Text Solution
|
- Henry's law constant for the solubility of nitrogen gas in water at 29...
Text Solution
|
- The freezing point (.^(@)C) of a solution containing 0.1 g of K(3)[Fe(...
Text Solution
|
- For a dilute solution containing 2.5 g of a non-volatile non-electroly...
Text Solution
|
- Consider separate solutions of 0.500 M C(2)H(5)OH(aq),0.100 M Mg(3)(PO...
Text Solution
|