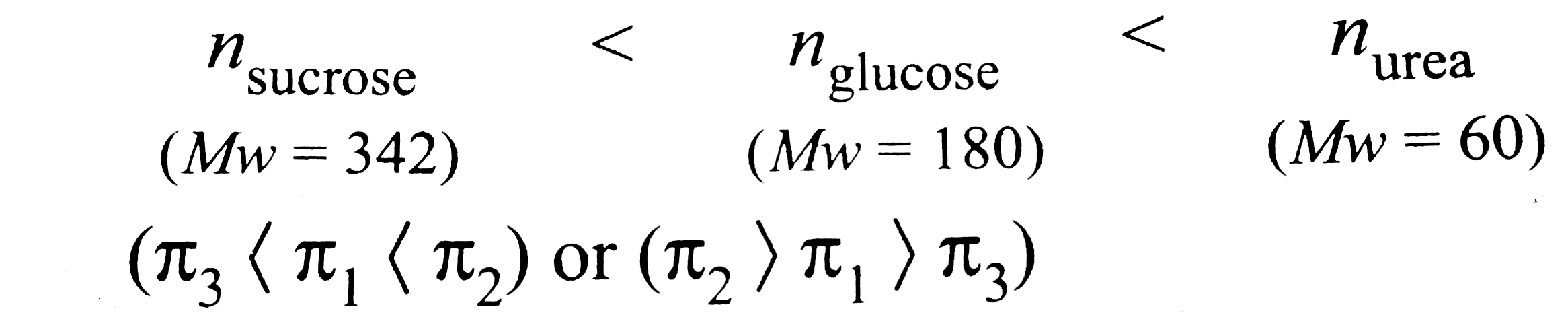A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SOLUTIONS-Ex 2.2 (Objective)
- An aqueous solution at -2.55^(@)C. What is its boiling point (K(b)^(H(...
Text Solution
|
- The relative decrease in VP of an aqueous glucose dilute solution is f...
Text Solution
|
- 10.0 g of glucose (pi(1)), 10.0 g of (urea(pi(2)), and 10.0 g of sucro...
Text Solution
|
- 0.6gof a solute is dissolved in 0.1litre of a solvent which develops a...
Text Solution
|
- A 5% solution of cane sugar (molecular weight =342) is isotonic with a...
Text Solution
|
- What mass of urea be dissolved in 171 g of water so as to decrease the...
Text Solution
|
- The vapour pressure at a given temperature of an ideal solution contai...
Text Solution
|
- Vapour pressure of a solution of 5gof non-electrolyte in 100gwater at ...
Text Solution
|
- The molal boiling point constant for water is0.513^(@)C kgmol^(-1). Wh...
Text Solution
|