Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SHORT ANSWER QUESTIONS|25 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|12 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise PROBLEMS|55 VideosD AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|33 VideosGENERAL PRINCIPLES OF MMETALLURGY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE SAQ|1 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ELECTROCHEMISTRY & CHEMICAL KINETICS-VERY SHORT ANSWER QUESTIONS
- Write the cell reaction taking plce in the cell CU((s))|Cu((aq))^(+...
Text Solution
|
- What is standard hydrogen electrode ?
Text Solution
|
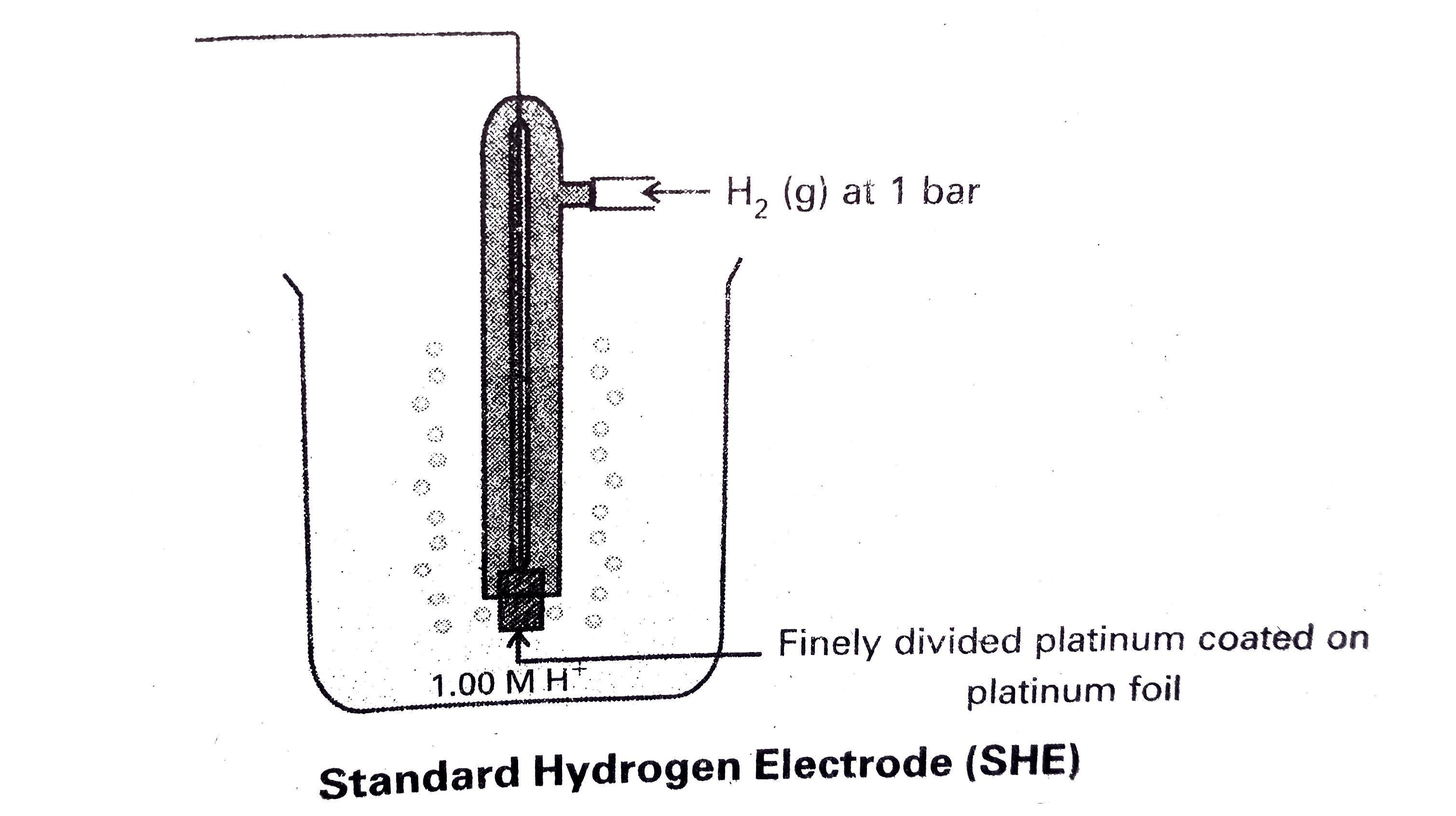
- Give a neat sketch of standard hydrogen electrode.
Text Solution
|
- What is Nernst equation ? Write the equation for an electrode with ele...
Text Solution
|
- A negative E^(0) indicates that the rodox couple is "" reducing couple...
Text Solution
|
- A positive E^(0) indicates that the redox couple is a weaker "" couple...
Text Solution
|
- Write the Nernst equation for the EMF of the cell Ni ((s))|Ni +((aq)...
Text Solution
|
- Write the cell reaction for which E(cell) =E(cell)^(0) -(RT)/(2F)ln ""...
Text Solution
|
- How is E^(0) cell related mathematically to the equilibrium constant K...
Text Solution
|
- How is Gibbs energy (G) related to the cell emf (E) mathematically ?
Text Solution
|
- Diffine conductivity of a material. Give its SI units.
Text Solution
|
- What is cell constant of a conductivity cell ?
Text Solution
|
- Define molar conducticity ^^(m) and how is it related to conductivity ...
Text Solution
|
- Give the mathematical equation which gives the variation of molar cond...
Text Solution
|
- State Kohlrausch's law of independent magration of ions.
Text Solution
|
- State Faraday's first law of electrolysis.
Text Solution
|
- State Faraday's seconed law of electroystis.
Text Solution
|
- What are the products obtainded at the platinum anode and the platinum...
Text Solution
|
- Give the products obtained at the platinum electrodes (cathode and ano...
Text Solution
|
- Give the chemical equation that represents the reduction of liquid wat...
Text Solution
|