Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise SHORT ANSWER QUESTIONS|25 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|12 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise PROBLEMS|55 VideosD AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|33 VideosGENERAL PRINCIPLES OF MMETALLURGY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE SAQ|1 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ELECTROCHEMISTRY & CHEMICAL KINETICS-VERY SHORT ANSWER QUESTIONS
- What are elementary reactions ?
Text Solution
|
- What are compelex reactions ? Name one complex reaction.
Text Solution
|
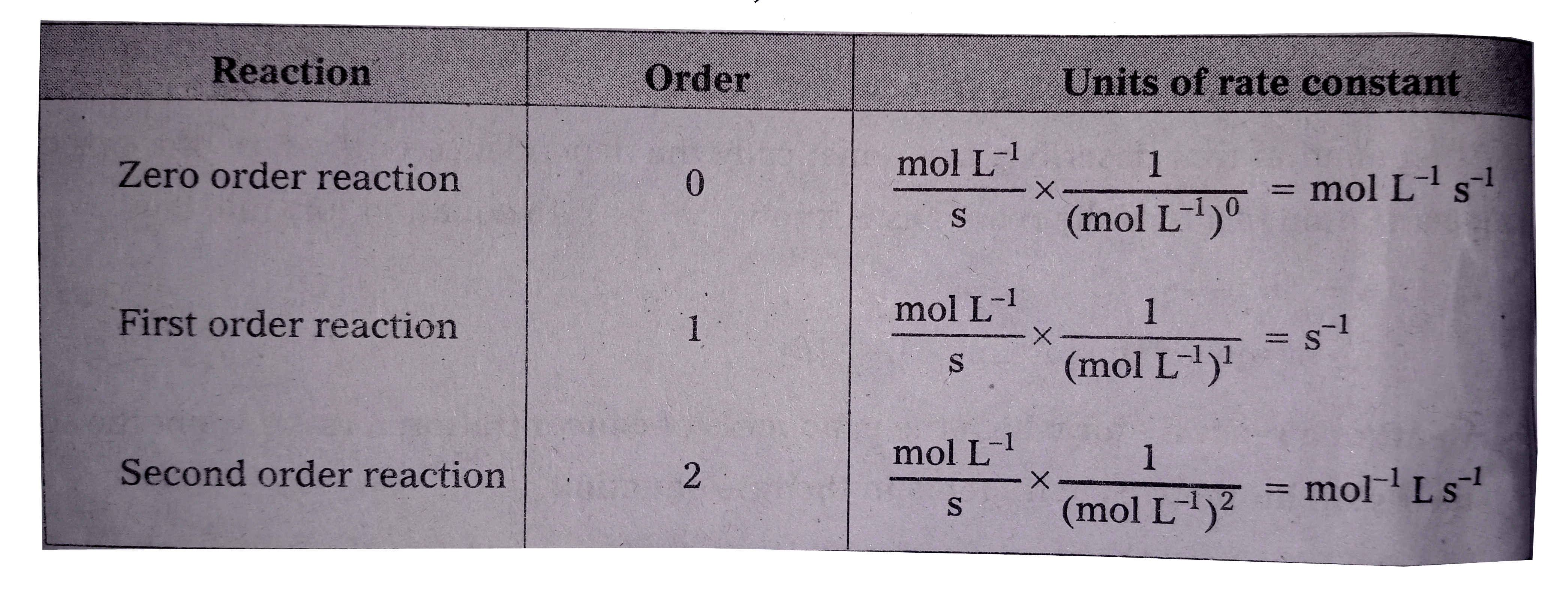
- Give the units of rate constants for Zero, first order and second orde...
Text Solution
|
- Define molecularity of a reaction, Illustrate with an example.
Text Solution
|
- What is rate determining step in a complex reaction ?
Text Solution
|
- Give the mechanism for the decomposition reacton of H(2)O(2) in alkali...
Text Solution
|
- Write the equation relating [R], [R](0) and reaction time 't' for a z...
Text Solution
|
- Drawn the graph that the concentration 'R', of the reactant and 't' th...
Text Solution
|
- Give two examples for zero order reaction.
Text Solution
|
- Write the intergrated equation for a fiest order reaction in terms of ...
Text Solution
|
- Give two examples for gaseous first order reactions.
Text Solution
|
- For the reaction A (g) to B(g) +C(g), write the intergrated rate equat...
Text Solution
|
- What is half-life of a reaction? Illustrate your answer with an exampl...
Text Solution
|
- Write the equation relating the half-life (t (1//2)) of a reaction and...
Text Solution
|
- Write the equation useful to calculate half-life (t(1//2)) values for ...
Text Solution
|
- What are pseudo first order reactions ? Give one example.
Text Solution
|
- Write the Arrhenius equation for the rate constant (k) of a reaction.
Text Solution
|
- By how many times the rate constant inhcreases for a rise of reaction ...
Text Solution
|
- Explain the term 'activation energy' of a reaction with a suitable dia...
Text Solution
|
- Write the equation which ralates th rate constants k(1)and k(2) at tem...
Text Solution
|