Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|12 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|24 VideosELECTROCHEMISTRY & CHEMICAL KINETICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise VERY SHORT ANSWER QUESTIONS|63 VideosD AND F- BLOCK ELEMENTS AND CO-ORDINATION COMPOUNDS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise INTEXT QUESTIONS|33 VideosGENERAL PRINCIPLES OF MMETALLURGY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise DAM SURE SAQ|1 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-ELECTROCHEMISTRY & CHEMICAL KINETICS-SHORT ANSWER QUESTIONS
- How is molar conductivity of an aqueous electrolyte solution measured ...
Text Solution
|
- Explain the varition of molar conductivity with the charge in the conc...
Text Solution
|
- State and explain Kohlrausch's law of indendent migration of ions.
Text Solution
|
- What is electrolysis ? Give Faraday's first law of electrolysis.
Text Solution
|
- What are the products obtained at the cathode and anode during the ele...
Text Solution
|
- What are primary and secondary batteries ? Give one example for each.
Text Solution
|
- What are the fuel cells ? How are they different from galvanic cells ?...
Text Solution
|
- What is metallic corrosion ? Explain it with respect to iron corrosion...
Text Solution
|
- Define average rate of a reaction. How is the rate of reaction express...
Text Solution
|
- What is rate equation ? How is it obtained ? Write the rate equations ...
Text Solution
|
- Define and explain the order of a reaction. How is it obtained exprime...
Text Solution
|
- What is "molecularity" of a reaction ? How is it different from the 'o...
Text Solution
|
- Derive the intergrate rate equation for a zero order rection.
Text Solution
|
- Derive an integrated rate equatin for a first order reaction.
Text Solution
|
- Derive an integrated rate equation in terms of total pressure (P) and ...
Text Solution
|
- What is half-life (t(1//2)) of a reaction ? Derive the equations for t...
Text Solution
|
- What is Arrhenius equation ? Derive an equation which describes the ef...
Text Solution
|
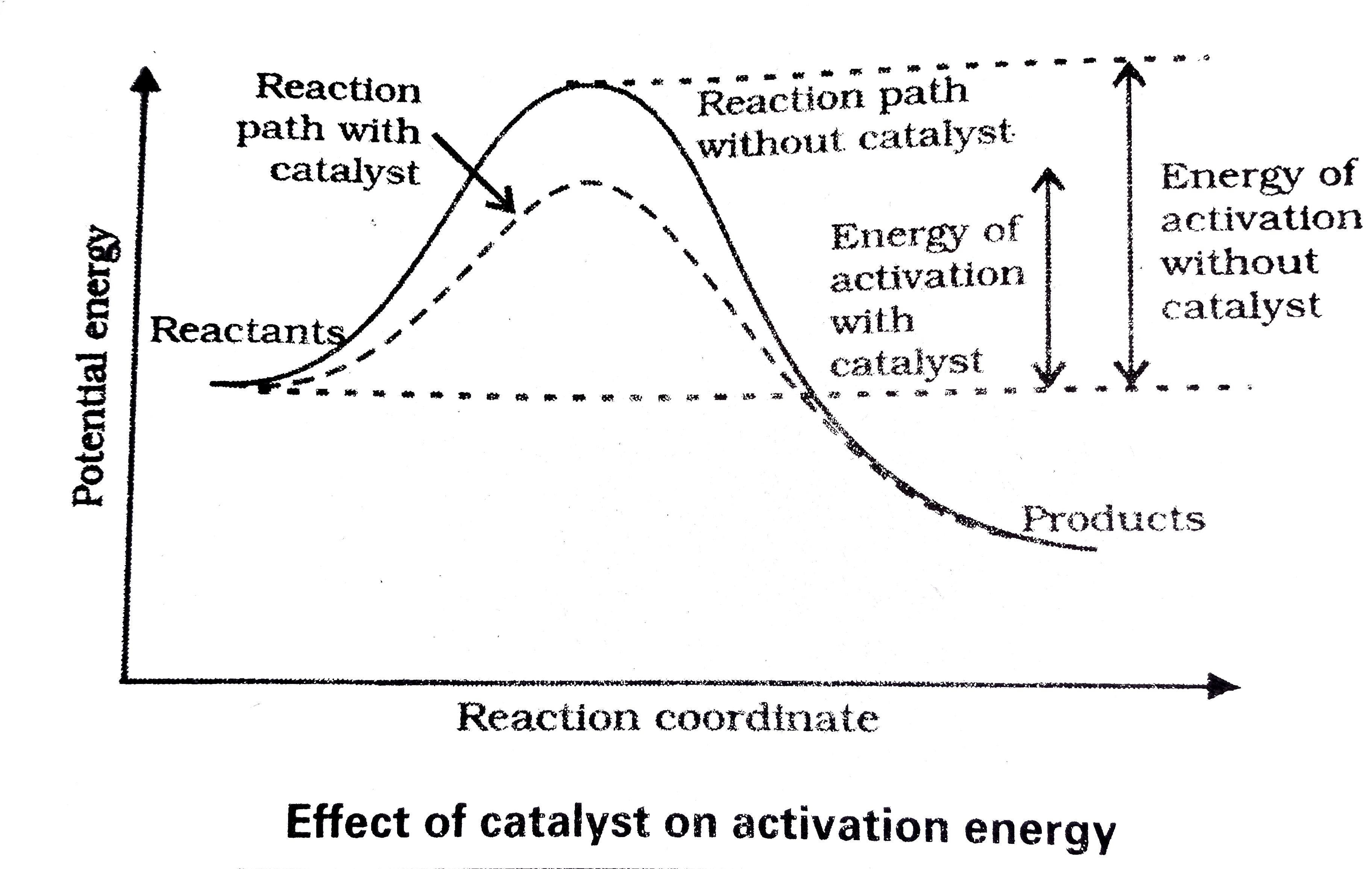
- Discuss the effect of catalyst on the kinetics of a chemical reaction ...
Text Solution
|
- Give a detailed account of the Collision theory of reaction rates of b...
Text Solution
|
- Explain the terms a) Activation energy (E(a)) b) Collision frequen...
Text Solution
|