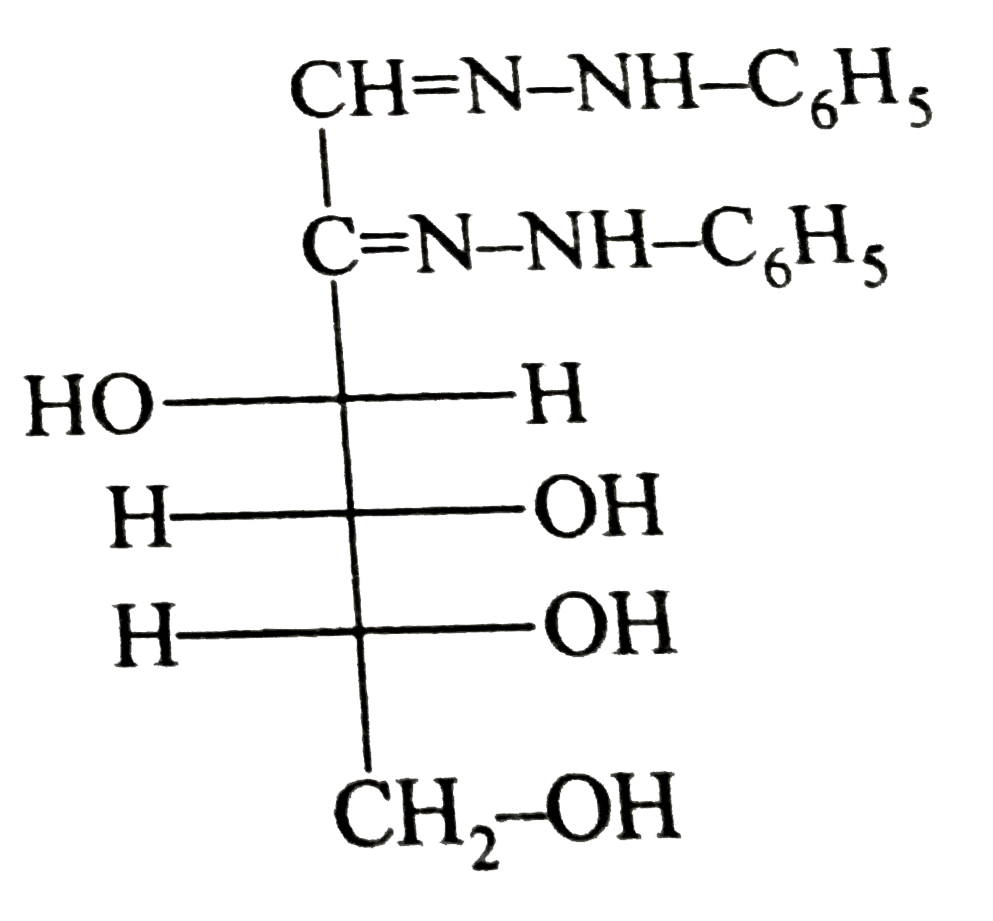A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-GENERAL ORGANIC CHEMISTRY-Exercise 2
- Observe these compound and give answer of following questions. Q. A ...
Text Solution
|
- Observe these compound and give answer of following questions. Q. Wh...
Text Solution
|
- Statement-I: per molecule of Glucose consumes two molecule of Ph-NH-NH...
Text Solution
|
- Statement-I: Solubility of an amino acid is maximum at isoelectric poi...
Text Solution
|
- Carbohydrates may be
Text Solution
|
- Select the correct statement-
Text Solution
|
- Select the incorrect statement-
Text Solution
|
- All sixteen possible aldohexose have been isolated or synthesised. Onl...
Text Solution
|
- Which is not correct about monosaccharides
Text Solution
|
- Which of the following is diasaccharides?
Text Solution
|
- Select the correct statement-
Text Solution
|
- Select the correct statement-
Text Solution
|
- Select the correct option.
Text Solution
|
- Amino acids are synthesised from
Text Solution
|
- Which of the following carbohydrates develops a deep blueback colour o...
Text Solution
|
- Which of the following are correct
Text Solution
|
- beta-D-(+) Glucopyranose is
Text Solution
|
- Which statement(s) is/are correct.
Text Solution
|
- Glycosidic linkage is present in
Text Solution
|
- Free aldehyde group can be produced on hydrolysis of which compound(s)...
Text Solution
|