Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROGEN
NCERT EXEMPLAR|Exercise MATCHING THE COLUMNS|4 VideosHYDROGEN
NCERT EXEMPLAR|Exercise ASSERTION AND REASON|2 VideosHYDROGEN
NCERT EXEMPLAR|Exercise LONG ANSWER TYPE QUESTIONS|8 VideosHYDROCARBONS
NCERT EXEMPLAR|Exercise Long Answer Type Questions|4 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
NCERT EXEMPLAR|Exercise Long Answer type question|6 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-HYDROGEN-SHORT ANSWER TYPE QUESTIONS
- Discuss briefly de- mineralisation of water by ion exchange resin.
Text Solution
|
- Molecular hydrides are classified as electron deficient, electron pre...
Text Solution
|
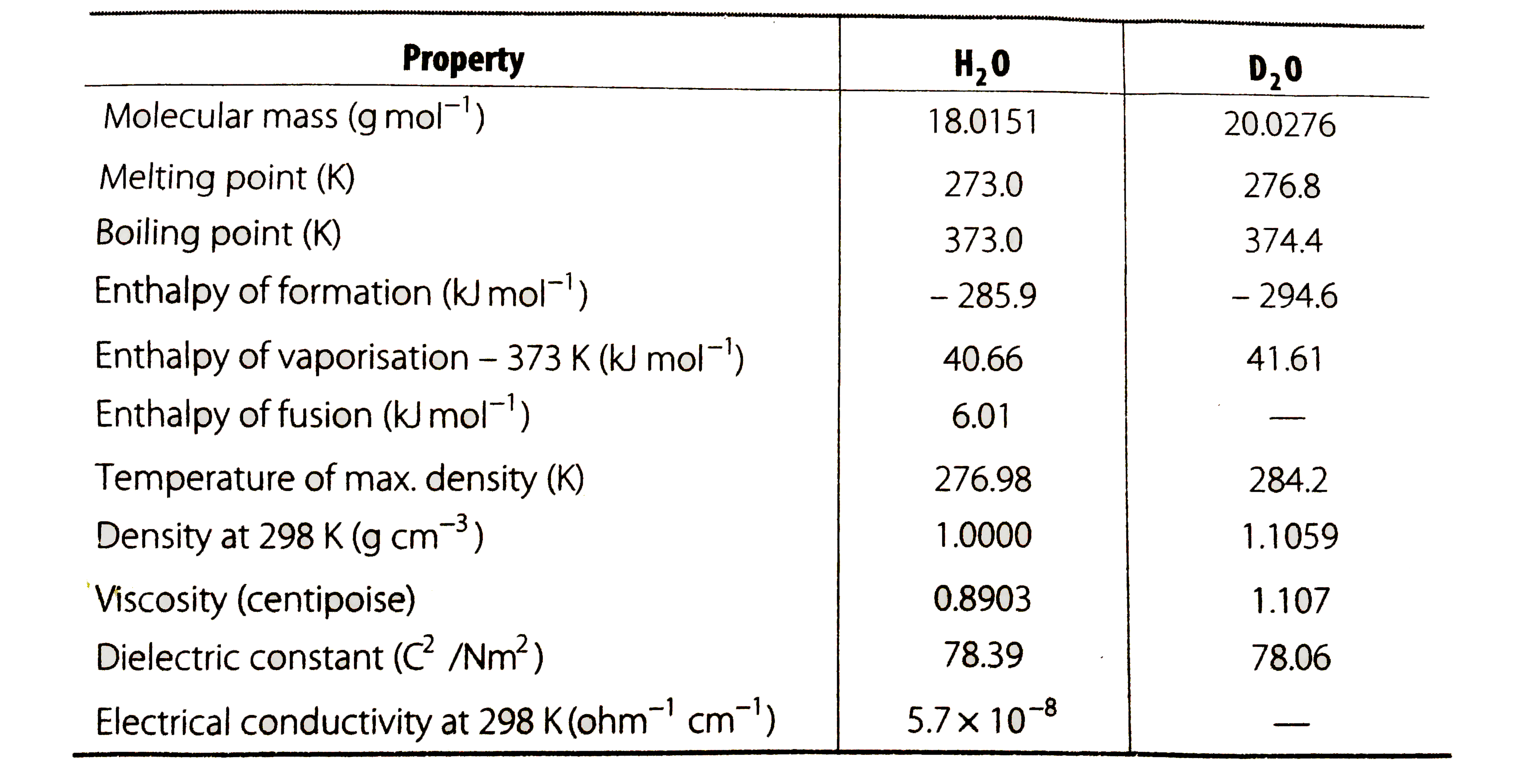
- How is heavy water prepared? Compare its physical properties with ...
Text Solution
|
- Write one chemical reactions for the preparation of D(2)O(2).
Text Solution
|
- Calculate the strenght of 5 volumes H(2)O(2) solution.
Text Solution
|
- (i) Draw the gas phase and solid phase structure of H(2)O(2). (ii...
Text Solution
|
- Melting point, enthaply of vaporisation and visvocsity data of H(2...
Text Solution
|
- Dihydrogen reacts with dioxygen (O(2)) to from water .Write the na...
Text Solution
|
- Explain why HCl is a gas and HF is a liquid ?
Text Solution
|
- When the first element of the periodic table is treated with diox...
Text Solution
|
- Rohan heard that instructions were given to the laboratory attend...
Text Solution
|
- Given reason why hydrogen resembles alkali metals ?
Text Solution
|
- Hydrogen generally form covalent compounds. Give reason
Text Solution
|
- Why is the ionisation enthalpy of hydrogen higher than that of sod...
Text Solution
|
- Basic pricinple of hyrogen economy is transpotation and storage of ...
Text Solution
|
- What is the importance of heavy water ?
Text Solution
|
- Write the Lewis structure of hydrogen peroxide .
Text Solution
|
- An acidic solution of hydrogen peroxide behaves as an oxidising ...
Text Solution
|
- With the help of suitable examples, explain the property of H(2)O(2...
Text Solution
|
- Why is water molecule polar ?
Text Solution
|