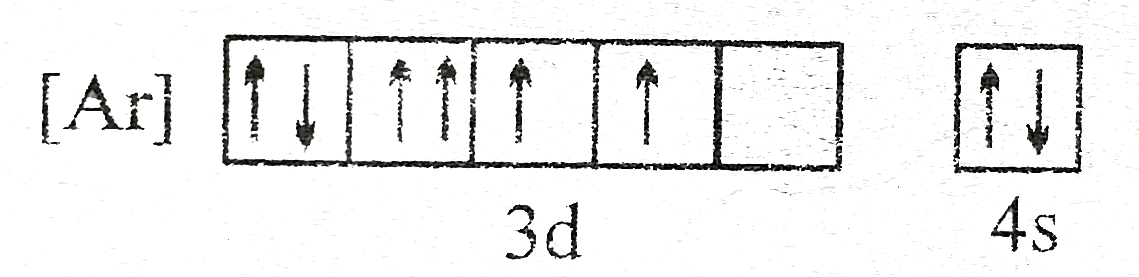A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
FIITJEE|Exercise MATRIX MATCH TYPE QUESTIONS|17 VideosATOMIC STRUCTURE
FIITJEE|Exercise SINGLE INTEGER TYPE QUESTIONS|8 VideosATOMIC STRUCTURE
FIITJEE|Exercise COMPEREHENSION-III|5 VideosALKYL AND ARYL HALIDES
FIITJEE|Exercise NUMERICAL BASED QUESTIONS|1 VideosCARBOHYDRATES, AMINO ACIDS, POLYMERS AND PRACTICAL ORGANIC CHEMISTRY
FIITJEE|Exercise EXERCISE|4 Videos
Similar Questions
Explore conceptually related problems