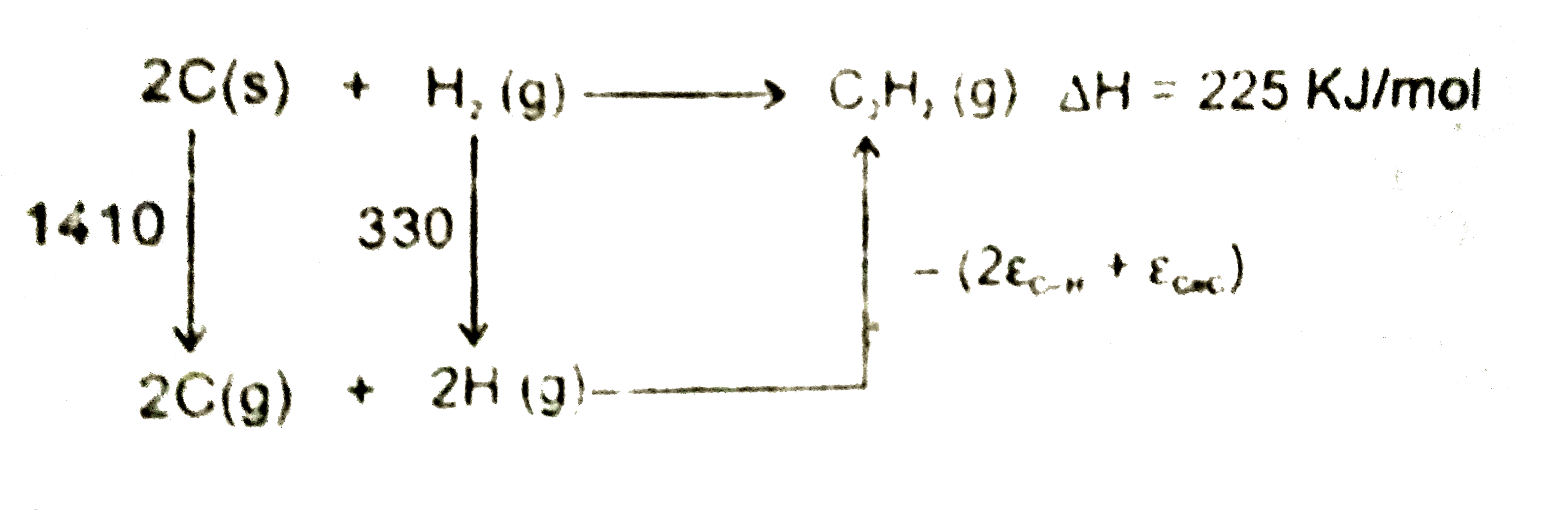A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-THERMODYNAMICS-exercise-3 Part-1 :(previous years)
- Which of the following equation gives the values of heat of formation ...
Text Solution
|
- In a constant volume calorimeter, 3.5 g of a gas with moleular weight ...
Text Solution
|
- The species which by definition has zero standard molar enthalpy of fo...
Text Solution
|
- The bond energy (in Kcal mol^(-)) of a C-C single bond is approximatel...
Text Solution
|
- Using the data provided, calculate the multiple bond energy (kJ mol^(-...
Text Solution
|
- The standard enthalpies fo formation of CO(2) (g), H(2) O (1), and glu...
Text Solution
|
- When 100mL of 1.0M HCl was mixed with 100 mL of 1.0 M NaOH in an insul...
Text Solution
|
- When 100mL of 1.0M HCl was mixed with 100 mL of 1.0 M NaOH in an insul...
Text Solution
|