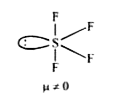
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
DISHA PUBLICATION|Exercise EXERCISE-1: CONCEPT BUILDER (TOPICWISE) (TOPIC 3: VSEPR Theory, VBT Theory and Hybridization)|36 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DISHA PUBLICATION|Exercise EXERCISE-1: CONCEPT BUILDER (TOPICWISE) (TOPIC 4: MOT and Hydrogen Bonding)|14 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
DISHA PUBLICATION|Exercise EXERCISE-1: CONCEPT BUILDER (TOPICWISE) (TOPIC 1: Electrovalent, Covalent and Coordinate Bonding)|17 VideosCHAPTERWISE NUMERIC/INTEGER ANSWER QUESTIONS
DISHA PUBLICATION|Exercise CHAPTER 28- BIOMOLECULES|10 VideosCHEMICAL KINETICS
DISHA PUBLICATION|Exercise EXERCISE 2 : CONCEPT APPLICATOR|30 Videos
Similar Questions
Explore conceptually related problems
DISHA PUBLICATION-CHEMICAL BONDING AND MOLECULAR STRUCTURE -EXERCISE-1: CONCEPT BUILDER (TOPICWISE) (TOPIC 2: Octet Rule, Resonance, Dipole Moment and Bond Polarity)
- A pair of compounds which has odd electrons in the group NO,CO,CIO,N(2...
Text Solution
|
- Point out incorrect statement about resonance
Text Solution
|
- In the cyanide ion, the formal negative charge is on :
Text Solution
|
- Among the following, the species having the smallest bond is
Text Solution
|
- The bond length of C = O bond in CO is 1.20 Å and in CO(2) it is 1.34 ...
Text Solution
|
- Which of the following would have a permanent dipole moment ?
Text Solution
|
- Dipole moment is shown by
Text Solution
|
- Which of the following salt shows maximum covalent character?
Text Solution
|
- Pauling's electronegativity values for elements are useful in predicti...
Text Solution
|
- Which of the following substances has the greatest ionic character ?
Text Solution
|
- Which bond angle, theta would result in the maximum dipole moment for ...
Text Solution
|
- polarisibility of halide ions increasing in the order
Text Solution
|
- If one assume linear structure instead of bent structure for water the...
Text Solution
|