Text Solution
Verified by Experts
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
OSWAAL PUBLICATION|Exercise TOPIC-2 (WERNER.S THEORY BONDING IN CO-ORDINATION COMPOUND , VBT, CFT AND IMPORTANCE OF CO-ORDINATION COMPOUNDS) (Very Short Answer Type Questions)|8 VideosCO-ORDINATION COMPOUNDS
OSWAAL PUBLICATION|Exercise TOPIC-2 (WERNER.S THEORY BONDING IN CO-ORDINATION COMPOUND , VBT, CFT AND IMPORTANCE OF CO-ORDINATION COMPOUNDS) (Short Answer Type Questions)|9 VideosCO-ORDINATION COMPOUNDS
OSWAAL PUBLICATION|Exercise TOPIC-1 (CO-ORDINATION COMPOUNDS AND THEIR PROPERTIES, IUPAC NOMENCLATURE OF MONONUCLEAR CO-ORDINATION COMPOUNDS (Short Answer Type Questions)|18 VideosCHEMISTY IN EVERYDAY LIFE
OSWAAL PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS-I|1 VideosD - BLOCK ELEMENTS F - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (F - BLOCK ELEMENT , LANTHANOIDS AND ACTINOIDS)(LONG ANSWER TYPES QUESTIONS)|6 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-CO-ORDINATION COMPOUNDS-TOPIC-1 (CO-ORDINATION COMPOUNDS AND THEIR PROPERTIES, IUPAC NOMENCLATURE OF MONONUCLEAR CO-ORDINATION COMPOUNDS (Long Answer Type Question-I)
- When a linkage isomerism is possible for co-ordination compounds ?
Text Solution
|
- Give the IUPAC name of [CoCl2 (NH3)4] Cl. Draw cis and trans isomers ...
Text Solution
|
- (a) What is coordination isomerism? Give an example.
Text Solution
|
- Write the IUPAC name of the complex: [Ag (NH3)2] [Ag(CN)2] .
Text Solution
|
- For [Co (en)3] Cl3 : Give the IUPAC name.
Text Solution
|
- For (Co(en)(3))Cl(3). Give the coordination number of the central me...
Text Solution
|
- For [Co (en)3] Cl3 : What type of stereoisomerism does it exhibit ?
Text Solution
|
- For the given complex [Co(NH(3))(5)Br]SO(4), write the IUPAC name and ...
Text Solution
|
- Which set of d-orbitals of a metal atom/ion experience more repulsion ...
Text Solution
|
- Give the IUPAC name of [Ti(H(2)O)(6)]^(3+). Draw cis and trans isomers...
Text Solution
|
- Mention the geometry and magentic property of tetracarbonylnickel comp...
Text Solution
|
- [Fe(CN)6]^(4-) and Fe(H2 O)6]^(2+) are different colours in dilute sol...
Text Solution
|
- The oxidation number of cobalt in K[Co(CO)4] is : (i) + 1 (ii) + 3 (ii...
Text Solution
|
- Write the IUPAC name of the complex [Cr(NH3)4 Cl2] Cl.
Text Solution
|
- What type of isomerism is exhibited by the complex [Co(en)3]^(3+) ? (...
Text Solution
|
- Why is [NiCl4]^(2-) paramagnetic but [Ni(CO)4] is diamagnetic ? (...
Text Solution
|
- How is double salt different from a complex ?
Text Solution
|
- Write IUPAC names of the following : (i) K3 [Fe(C2 O4)3] (ii) [Pt...
Text Solution
|
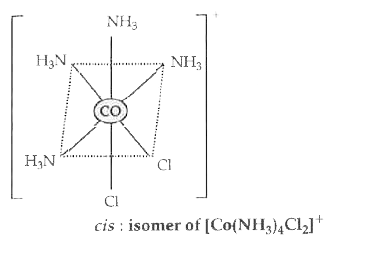
- Draw the structure of cis-isomer of [Co(NH3)4 Cl2]^+ .
Text Solution
|
- Write the optical isomers cis [PtCl2)en)2]^(2+).
Text Solution
|