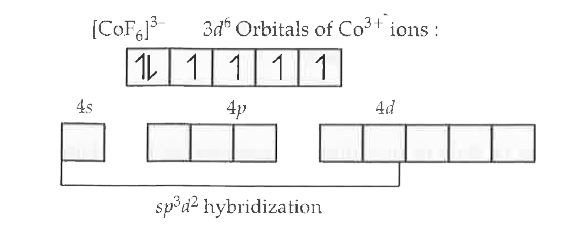
Text Solution
Verified by Experts
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
OSWAAL PUBLICATION|Exercise TOPIC-2 (WERNER.S THEORY BONDING IN CO-ORDINATION COMPOUND , VBT, CFT AND IMPORTANCE OF CO-ORDINATION COMPOUNDS) (Very Answer Type Questions)|19 VideosCO-ORDINATION COMPOUNDS
OSWAAL PUBLICATION|Exercise TOPIC-2 (WERNER.S THEORY BONDING IN CO-ORDINATION COMPOUND , VBT, CFT AND IMPORTANCE OF CO-ORDINATION COMPOUNDS) (Very Short Answer Type Questions)|8 VideosCHEMISTY IN EVERYDAY LIFE
OSWAAL PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS-I|1 VideosD - BLOCK ELEMENTS F - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (F - BLOCK ELEMENT , LANTHANOIDS AND ACTINOIDS)(LONG ANSWER TYPES QUESTIONS)|6 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-CO-ORDINATION COMPOUNDS-TOPIC-2 (WERNER.S THEORY BONDING IN CO-ORDINATION COMPOUND , VBT, CFT AND IMPORTANCE OF CO-ORDINATION COMPOUNDS) (Short Answer Type Questions)
- Mention any two characteristics of bonding molecular orbitals.
Text Solution
|
- State EAN rule for co-ordination compounds.
Text Solution
|
- What are the limitation of VBT ?
Text Solution
|
- What are t(2g) and eg orbitals ?
Text Solution
|
- What are the demerits of Werner's theory?
Text Solution
|
- Give the significance of d^4 ions in octahedral field.
Text Solution
|
- Using valence bond theory explain geometry, hybridisation and magnetic...
Text Solution
|
- Explain the Crystal field splitting is an octahedral field.
Text Solution
|
- What is spectrochemical series ? Explain the difference between a weak...
Text Solution
|