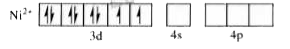A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 3 ( Linked Comprehension type questions Passage - 1:)|4 VideosCO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 3 ( Linked Comprehension type questions Passage - II:)|3 VideosCO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (Match the following questions )|8 VideosCHEMISTRY IN EVERYDAY LIFE
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (PRACTICE SHEET -2 (INTEGER ANSWER TYPE QUESTIONS))|7 VideosCOMPLEX COMPOUNDS
AAKASH SERIES|Exercise PRACTICE EXERCISE|45 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CO-ORDINATION COMPOUNDS-PRACTICE SHEET - 3 (Single or more than one option questions)
- The coordination number and oxidation number of 'x' in the following, ...
Text Solution
|
- The ligand called pi-acid is
Text Solution
|
- In Fe(CO)5 , Fe-C bond possess
Text Solution
|
- The hybridisation of central metal ion and shape of Wilkinson's cataly...
Text Solution
|
- Which of the following has dodechahedral geometry
Text Solution
|
- Which of the following organometallic compound in sigmma and pi bonded
Text Solution
|
- All the following complex ions are found to be paramagnetic P=[FeF(6...
Text Solution
|
- Which of the following statements in most likely to be incorrect
Text Solution
|
- What will be the theoritical value of spin only magnetic moment when F...
Text Solution
|
- Among the following, the species with tetrahedral geometry ?
Text Solution
|
- Which of the following statements are correct about stability of chela...
Text Solution
|
- Which of the following are paramagnetic
Text Solution
|
- Which of the following statements about Fe[(H(2)O)(5)NO]^(2-) are corr...
Text Solution
|
- [Fe(en)(2)(H(2)O)(2)]^(2+)+ento complex (x). The correct statement abo...
Text Solution
|
- The correct statements among the following are.
Text Solution
|
- Among the following, the ions having same magnetic moment are
Text Solution
|