Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY SUPPLEMENTARY EXAM QUESTION PAPER JUNE -2019
SUNSTAR PUBLICATION|Exercise PART-C|15 VideosII PUC CHEMISTRY SUPPLEMENTARY EXAM QUESTION PAPER JUNE -2019
SUNSTAR PUBLICATION|Exercise PART-D|34 VideosII PUC CHEMISTRY SUPPLEMENTARY EXAM QUESTION PAPER JUNE -2019
SUNSTAR PUBLICATION|Exercise PART-D|34 VideosII PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2016)
SUNSTAR PUBLICATION|Exercise PART - D|25 VideosK - CET - CHEMISTRY - 2015
SUNSTAR PUBLICATION|Exercise MCQs|60 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY SUPPLEMENTARY EXAM QUESTION PAPER JUNE -2019-PART-B
- Which type of extrinsic semiconductor is formed when silicon is dopped...
Text Solution
|
- Draw a neat labeled diagram of Standard Hydrogen Electrode (SHE). Writ...
Text Solution
|
- Name any two factors affecting the rate of a reaction.
Text Solution
|
- Zr and Hf have almost identical atomic radii. Give reason.
Text Solution
|
- (a)Zr and Hf have almost identical radii : Give reason. (b)Name the ga...
Text Solution
|
- How does phenol react with Conc.HNO3? Give equation.
Text Solution
|
- Explain Cannizzaro reaction with an example.
Text Solution
|
- What is the role of the following chemicals in food? Sodium benzoate
Text Solution
|
- What is the role of the following chemicals in food? Saccharin
Text Solution
|
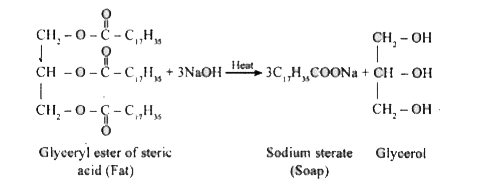
- What is saponification? Write the equation to get sodium stearate by t...
Text Solution
|