Text Solution
Verified by Experts
Topper's Solved these Questions
II PUC CHEMISTRY SUPPLEMENTARY EXAM QUESTION PAPER JUNE -2019
SUNSTAR PUBLICATION|Exercise PART-D|34 VideosII PUC CHEMISTRY SUPPLEMENTARY EXAM QUESTION PAPER JUNE -2019
SUNSTAR PUBLICATION|Exercise PART-B|10 VideosII PUC CHEMISTRY (SUPPLEMENTARY EXAM QUESTION PAPER JULY - 2016)
SUNSTAR PUBLICATION|Exercise PART - D|25 VideosK - CET - CHEMISTRY - 2015
SUNSTAR PUBLICATION|Exercise MCQs|60 Videos
Similar Questions
Explore conceptually related problems
SUNSTAR PUBLICATION-II PUC CHEMISTRY SUPPLEMENTARY EXAM QUESTION PAPER JUNE -2019-PART-C
- With a neat labelled diagram,describe the extraction of aluminium by H...
Text Solution
|
- In the extraction of Aluminium by Hall- Herault process: Give the e...
Text Solution
|
- In the extraction of Aluminium by electrolysis Role of cryolite
Text Solution
|
- For the manufacture of Ammonia by Haber’s process, write the flow char...
Text Solution
|
- Complete the following equation: 4Al + 3O2 to
Text Solution
|
- a) Complete the following equations: i) PbS((s))+4O(3(g))rarr ii) ...
Text Solution
|
- Complete the following equations: C + Conc. 2H 2SO 4 to'
Text Solution
|
- Mention any two resons for anomalous behaviour of Fluorine.
Text Solution
|
- Write the structure of perchloric acid (HClO4)
Text Solution
|
- (a) Calculate the spin only magnetic moment of Ti^(3+)ion (Atomic numb...
Text Solution
|
- (a) Calculate the spin only magnetic moment of Ti^(3+)ion (Atomic numb...
Text Solution
|
- Write the balanced equations involved in the preparation of potassium ...
Text Solution
|
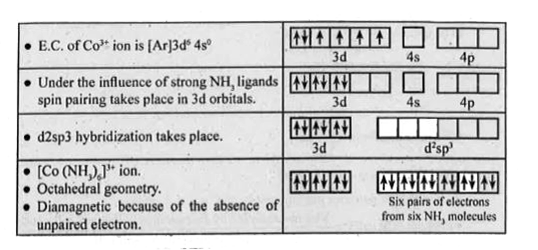
- Explain the hybridization, geometry and magnetic property of [Co(NH3)6...
Text Solution
|
- For a given complex [Co(NH3)5 NO2 ] Cl2. Write its IUPAC name and Link...
Text Solution
|
- Which set of d-orbitals of a metal atom/ion experience more repulsion ...
Text Solution
|