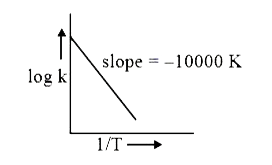Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
JEE MAIN 2021
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY (SECTION-A)|20 VideosJEE MAIN 2021
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY (SECTION-B)|10 VideosJEE MAIN 2021
JEE MAINS PREVIOUS YEAR|Exercise SECTION-A|100 VideosJEE MAIN
JEE MAINS PREVIOUS YEAR|Exercise CHEMISTRY|150 VideosJEE MAIN 2022
JEE MAINS PREVIOUS YEAR|Exercise Question|561 Videos
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR-JEE MAIN 2021-SECTION-B
- The reaction of cyanamide, NH(2)CN(s), with dioxygen was carried out i...
Text Solution
|
- Using the provide information in the following paper chromatogarm ...
Text Solution
|
- For the reaction aA+bBrarrcC+dD, the plot of log k vs 1/T is given bel...
Text Solution
|
- 0.4 g mixture of NaOH, Na2CO3, and some inert N impurities was first t...
Text Solution
|
- HC-=CH overset(Red Hot Fe) underset (AlCl3) to [X] overset (CO,HCl//Al...
Text Solution
|
- The ionization enthalpy of Na^+ formation from Nad is 495.8 kJ mol^(-1...
Text Solution
|
- For a chemical reaction A+ B hArr C + D (Deltar H^0 = 80 KJ "mol"^(-...
Text Solution
|
- Find the significant figure in 50000.020 xx 10^(-3)
Text Solution
|
- An exothermic reaction X to Y has an activation energy 30 KJ "mol"^(-1...
Text Solution
|
- Consider the following reaction MnO4^(-) + 8 H^(+) 5e^(-) to Mn^(...
Text Solution
|
- For a real gas following vander waal equation is obtained P(Vm-b) =RT ...
Text Solution
|
- A homogeneous ideal gaseous reaction AB(2(g)) hArr A((g)) + 2B((g)) ...
Text Solution
|
- Dichromate ion is treated with base, the oxidation number of Cr in the...
Text Solution
|
- 224 mL of SO(2(g)) at 298 K and 1 atm is passed through 100 mL of 0.1 ...
Text Solution
|
- 3.12 g of oxygen is adsorbed on 1.2 g of platinum metal. The volume of...
Text Solution
|
- Number of bridging CO ligands in [Mn2 (CO)(10)] is .
Text Solution
|
- The total number of amines among the following which can be synthesize...
Text Solution
|
- Among the following allotropic forms of sulphur , the number of allotr...
Text Solution
|
- The formula of a gaseous hydrocarbon which requires 6 times of its own...
Text Solution
|
- The volume occupied by 4.75g of acetylene gas at 50^(@)C and 740 mmH...
Text Solution
|