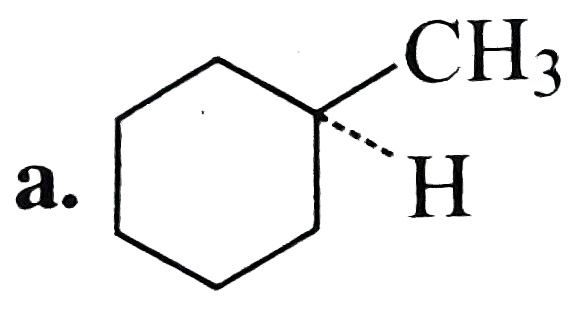
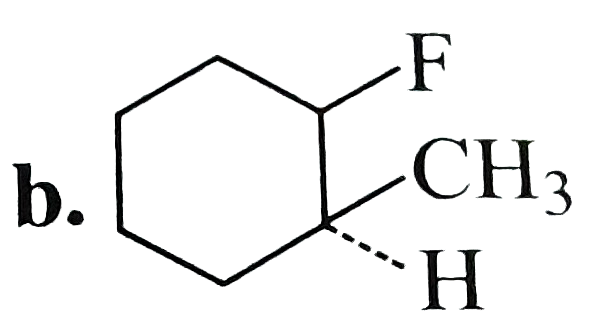
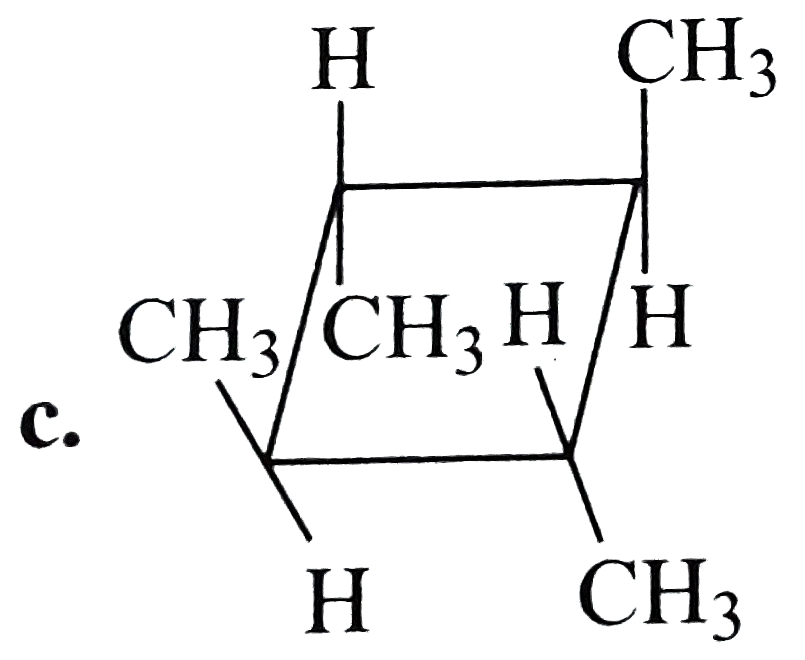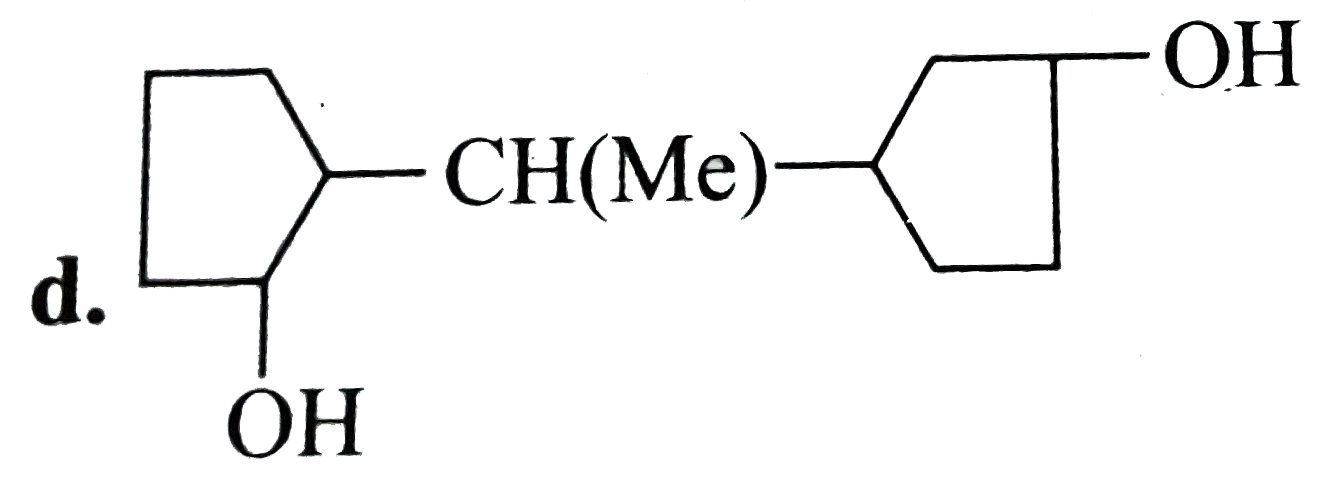A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Single correct answer type|13 VideosISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Single correct answer type (Exercise)|65 VideosISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise (Linked Comprehension Type)Exercise|13 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|28 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Chemical Equilibrium|73 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ISOMERISM-Multiple choice questions (Exercise)
- Which of the following will show geometrical isomerism ?
Text Solution
|
- Which of the following are not resolvable?
Text Solution
|
- Which of the following are resolvable ?
Text Solution
|
- Which of the following is/are optically active?
Text Solution
|
- The compounds are optically inactive because
Text Solution
|
- The stable conformer (s) of trans-1-4- dimethyl] cyclohexane is/are:
Text Solution
|
- The stable conformer (s) of cis-cyclohexane 1-3- diol is/are:
Text Solution
|
- Which form (s) of cyclohexane is/are free from angle strain?
Text Solution
|
- Which of the following statements is/are wrong, about the more stabili...
Text Solution
|
- Which of the following methods are used for resolution ?
Text Solution
|
- According to Baeyer's strain theory, which of the following is/are mos...
Text Solution
|
- The angle strain in cyclohexane is :
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- Which of the following statement regarding 1,2- dimethylcyclopropane (...
Text Solution
|
- Which of the following statements regarding 1,3- dimethyl cyclobutane ...
Text Solution
|
- Which of the following statements regarding 1,3- dimethl cyclobutane i...
Text Solution
|
- Which of the following statements regarding 1,2-dimethyl cyclo-pentane...
Text Solution
|
- The configuration of sugars is related to glyceraldehyde and that of a...
Text Solution
|
- Which of the following statement are true?
Text Solution
|
- Which of the following statements are correct?
Text Solution
|