Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise SECTION-H|3 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise SECTION-I|9 VideosTHERMODYNAMICS
AAKASH INSTITUTE ENGLISH|Exercise SECTION-F|3 VideosTHE SOLID STATE
AAKASH INSTITUTE ENGLISH|Exercise Assignment (SECTION - D) (ASSERTION-REASON TYPE QUESTION)|20 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-THERMODYNAMICS-SECTION-G
- The maximum work done when pressure of n moles of H(2) was reduced fr...
Text Solution
|
- One mole of an ideal monoatomic gas expands reversibly and adiabatical...
Text Solution
|
- For a reversible reaction A hArr B. Find (log(10)K)/(10) at 2727^(@)C...
Text Solution
|
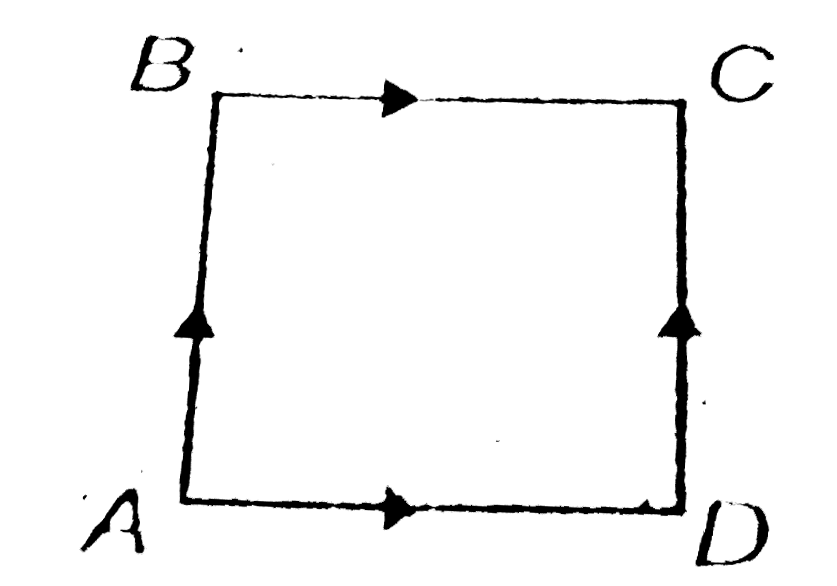
- When a system is taken from A to C through path ABC, 10 J of heat flow...
Text Solution
|
- The chemical reaction : A rarr P, Delta H^(@) = 2.8 kJ is spontaneous ...
Text Solution
|