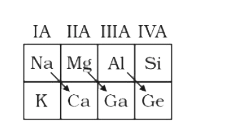
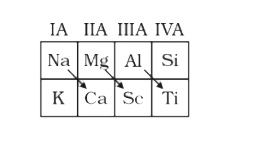
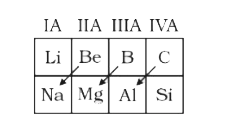A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise LEVEL -2 Numerical Value Type for JEE Main|13 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise JEE MAIN (ARCHIVE)|13 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise LEVEL -1|75 VideosCHEMICAL THERMODYNAMICS
VMC MODULES ENGLISH|Exercise IN - CHAPTER EXERCISE - L|10 VideosELECTROCHEMISTRY
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES -LEVEL -2
- The energy required to pull the most loosely bound electron form an at...
Text Solution
|
- The energy required to pull the most loosely bound electron form an at...
Text Solution
|
- Select which is correct representation for diagonal relationships in p...
Text Solution
|
- Consider the following reaction: (i) O((g))+e^(-) to O((g))^(-),Delt...
Text Solution
|
- What is the atomic number of the element with the maximum number of u...
Text Solution
|
- O(g) + 2e^(-) to O^(2-)(g) , DeltaH(eg) = 744.7 kJ/mole . The positive...
Text Solution
|
- Consider the order O^(2-) lt F^(+) lt Na^(+) lt Mg^(3+). Then correct ...
Text Solution
|
- How do the energy gaps between successive electron energy levels in an...
Text Solution
|
- Which of the following properties of the alkaline earth metals increas...
Text Solution
|
- What would be the atomic number of the last member of the halogen grou...
Text Solution
|
- Which of the following process requires the largest amount of energy ?
Text Solution
|
- In which of the following pair, both the species are isoelectronic but...
Text Solution
|
- The correct order of ionic size of N^(3-), Na^(+), F^(-), Mg^(2+) and ...
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- Which of the following is the wrong statement?
Text Solution
|
- IE(1) and IE(2) of Mg are 178 and 348 kcal mol^(-1). The energy requir...
Text Solution
|
- X((g)) to X((g))^(+) +e^(-), " "DeltaH=+720kJ" "mol^(-1) C...
Text Solution
|
- Consider the following changes :- M((s)rarrM((g))" ""....."(a) ...
Text Solution
|
- Consider the following values of I.E.(eV) for elements W and X: {:("...
Text Solution
|
- Which is the correct order of ionization energies?
Text Solution
|