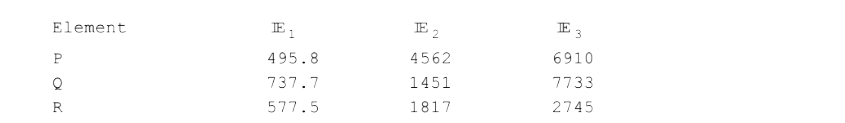A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise LEVEL -2 Numerical Value Type for JEE Main|13 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise JEE MAIN (ARCHIVE)|13 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise LEVEL -1|75 VideosCHEMICAL THERMODYNAMICS
VMC MODULES ENGLISH|Exercise IN - CHAPTER EXERCISE - L|10 VideosELECTROCHEMISTRY
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES -LEVEL -2
- Consider the following ionisation reactions: If monovalent positi...
Text Solution
|
- The incorrect statement is:
Text Solution
|
- First three ionisation energies (in kJ/mol) of three representative el...
Text Solution
|
- Which of the following statement is correct regarding following proces...
Text Solution
|
- The correct order of increasing electron affinity of the following ele...
Text Solution
|
- The second electron gain enthalpies (in kJ mol^(-1)) of oxygen and sul...
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Which one of the following statements is incorrect?
Text Solution
|
- The formation of the oxide ion O^(2-) (g) requires first an exothermic...
Text Solution
|
- In which of the following processes, heat is neither absorbed nor rele...
Text Solution
|
- The electron affinity of the following elements can be arranged:
Text Solution
|
- In which of the following arrangements , the order is not correct acco...
Text Solution
|
- Which of the following statements is/are wrong?
Text Solution
|
- The stability of ions of Ge Sn and Pb will be in the order .
Text Solution
|
- Select the correct statement(s)/order(s):
Text Solution
|
- In the compound M – O – H, the M – O bond will be broken if:
Text Solution
|
- Aqueou solutions of two compounds M-O-H and M'-O-H have been prepared ...
Text Solution
|
- Consider the following ionization stesps: M(g) ot M^(+)(g)+e^(-)," ...
Text Solution
|
- Consider the following statements : (I) The radius of an anion is la...
Text Solution
|
- Which of the following order is correct for the property mentioned in ...
Text Solution
|