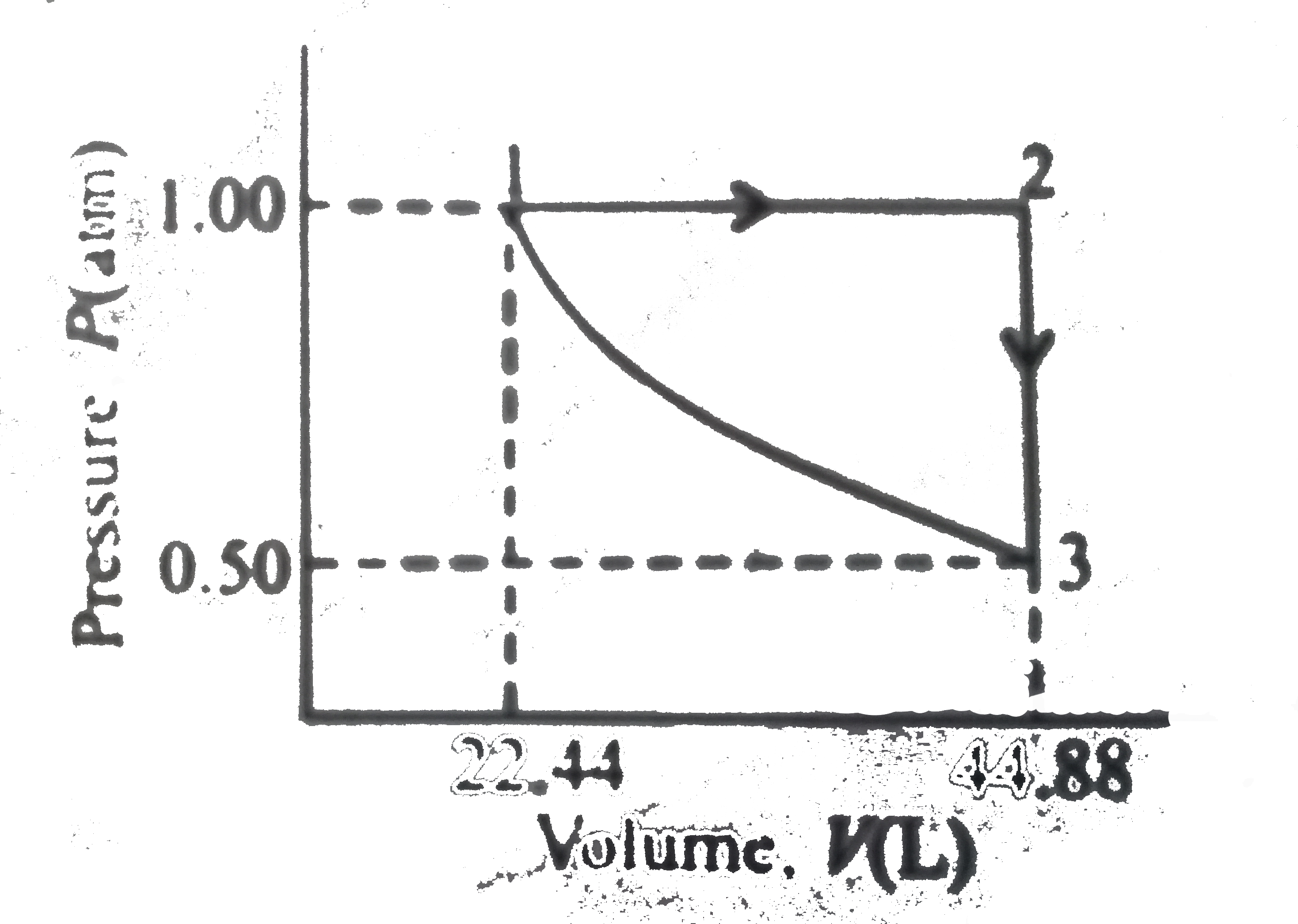
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-2 (NUMERICAL VALUE TYPE)|15 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise JEE (MAIN ARCHIVE)|32 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-1|75 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|31 VideosTHERMODYNAMICS & THERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise Impeccable|48 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THERMODYNAMICS-LEVEL-2
- What would be the heat absorbed by the system in the cyclic process?
Text Solution
|
- A sample consisting of 1 mole of a mono-atomic perfect gas (C(v) = 3/2...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- A sample consisting of 1mol of a mono-atomic perfect gas (C(V) = (3)/(...
Text Solution
|
- The enthalpy of vaporization of chloroform is 29.4 kJmol^(-1) at its n...
Text Solution
|
- Combustion of sucuose is used by aeroic organisms for providing energy...
Text Solution
|
- The freezing of any liquid to a solid is expected to have:
Text Solution
|
- Industrial acetylene gas (ethyne: C(2)H(2)) is made by the high temper...
Text Solution
|
- Which one of the following statements is true
Text Solution
|
- Calculate the standard free energy change for the formation of methane...
Text Solution
|
- Given: Delta(f)H^(@) (kJ//mol) " " S(m)^(@)(J//K mol) {:( C Cl(4)(...
Text Solution
|
- Calculate the change in molar Gibbs energy of carbon dioxide gas at 20...
Text Solution
|
- Calculate Delta(r)S("sys")^(@) for the following reaction at 373 K: ...
Text Solution
|
- A certain process releases 64.0 kJ of heat, which is transferred to th...
Text Solution
|
- Which of the following is (are) correct?
Text Solution
|
- When ice melts at 1^(@)C :
Text Solution
|
- The standard enthalpy of formation of gaseous H(2)O at 298 K is -241.8...
Text Solution
|
- Which statements in each of the following paris would you except to ha...
Text Solution
|
- DeltaH for solid to liquid transitions for protein A and B are 2.73 kc...
Text Solution
|