A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-2 (NUMERICAL VALUE TYPE)|15 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise JEE (MAIN ARCHIVE)|32 VideosTHERMODYNAMICS
VMC MODULES ENGLISH|Exercise LEVEL-1|75 VideosTHERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE ADVANCED (ARCHIVE)|31 VideosTHERMODYNAMICS & THERMOCHEMISTRY
VMC MODULES ENGLISH|Exercise Impeccable|48 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THERMODYNAMICS-LEVEL-2
- Which of the following is (are) correct?
Text Solution
|
- When ice melts at 1^(@)C :
Text Solution
|
- The standard enthalpy of formation of gaseous H(2)O at 298 K is -241.8...
Text Solution
|
- Which statements in each of the following paris would you except to ha...
Text Solution
|
- DeltaH for solid to liquid transitions for protein A and B are 2.73 kc...
Text Solution
|
- For the auto-ionization of water at 25^(@)C, H(2)O(l)iff H^(+)(aq)+OH^...
Text Solution
|
- For a particular reversible reaction at temperature T, DeltaH and Delt...
Text Solution
|
- Which of the following reactions defines DeltaH(f)^(@) ?
Text Solution
|
- The direct conversion of A or B is difficult, hence it is carried out ...
Text Solution
|
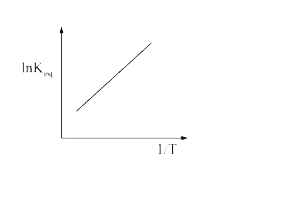
- A schematic plot of ln K(eq) versus inverse of temperature for a react...
Text Solution
|
- An endotthermic reaction is non-spontaneous at freezing point of water...
Text Solution
|
- Select the correct statement(s) about entropy S
Text Solution
|
- A particular reaction at 27^(@)C for which Delta H gt 0 and Delta S gt...
Text Solution
|
- For a given reaction, DeltaH = 35.5 kJ mol^(-1) and Delta S = 83.6 JK...
Text Solution
|
- Standard entropies of X(2), Y(2) and XY(3) are 60, 40 and 50 JK^(–1) m...
Text Solution
|
- The incorrect expression among the following is
Text Solution
|
- A reaction has DeltaH=-33kJ and DeltaS=-58J//K. This reaction would be...
Text Solution
|
- Animals operate under conditons of constant pressure and most of the p...
Text Solution
|
- 10 g of argon is compressed isothermally and reversibly at a temperatu...
Text Solution
|
- 4x^2-9=(px+t)(px-t) In the equation above, p and t are constants. Wh...
Text Solution
|