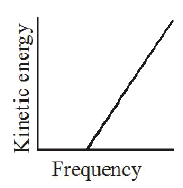
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURE OF ATOM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-C|15 VideosSTRUCTURE OF ATOM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-D|10 VideosSTRUCTURE OF ATOM
VMC MODULES ENGLISH|Exercise IN-CHAPTER EXERCISE-A|10 VideosSTOICHIOMETRY-II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|43 VideosSURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE|9 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-STRUCTURE OF ATOM-IN-CHAPTER EXERCISE-B
- According to Einstein's photoelectric equation, the graph between the ...
Text Solution
|
- If two pieces of caesium metal are supplied with energy hv(1) and hv(2...
Text Solution
|
- If lambda(0) is the threshold wavelength for photoelectric emission. ...
Text Solution
|
- If the threshold wavelength (lambda(0)) for ejection of electron from...
Text Solution
|
- A certain metal when irradiated by light (v=3.2xx10^(16)Hz) emits phot...
Text Solution
|
- The frequency of incident light falling on a photosensitive metal plat...
Text Solution
|
- The work function of a metal is 3.4 eV. A light of wavelength 3000Å is...
Text Solution
|
- The work function of a metal is 4.2 eV. If radiations of 2000Å fall o...
Text Solution
|
- Which of the following phenomenon could not be explained by wave natur...
Text Solution
|