Text Solution
Verified by Experts
Topper's Solved these Questions
SOLUTIONS
CENGAGE CHEMISTRY|Exercise Exercises (Linked Comprehension)|58 VideosSOLUTIONS
CENGAGE CHEMISTRY|Exercise Exercises (Multiple Correct)|25 VideosSOLUTIONS
CENGAGE CHEMISTRY|Exercise Ex 2.3 (Objective)|9 VideosSOLID STATE
CENGAGE CHEMISTRY|Exercise Ex 1.2 (Objective)|9 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY|Exercise Archives Subjective|2 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-SOLUTIONS-Solved Examples
- CNS^(ө) ions give red colour with Fe^(3+) ions in aqueous solution as:...
Text Solution
|
- At 17^(@)C, the osmotic pressure of sugar solution is 580 torr. The so...
Text Solution
|
- Among the following the solution which shows the lowest osmotic pressu...
Text Solution
|
- A 0.1 M solution of glucose (molecular weight 180 g "mol"^(-1)) and a...
Text Solution
|
- If the radiator of an automobile contains 12 L of water, how much woul...
Text Solution
|
- If the boiling point of an aqueous solution is 100.1^(@)C, what is its...
Text Solution
|
- The K(sp) (25^(@)C) of sparingly soluble salt XY(2)(s) is 3.56 xx 10^(...
Text Solution
|
- 0.5 g KCl was dissolved in 100 g water, and the solution, originally a...
Text Solution
|
- A 0.001 molal solution of a complex represented as Pt(NH(3))(4)Cl(4) i...
Text Solution
|
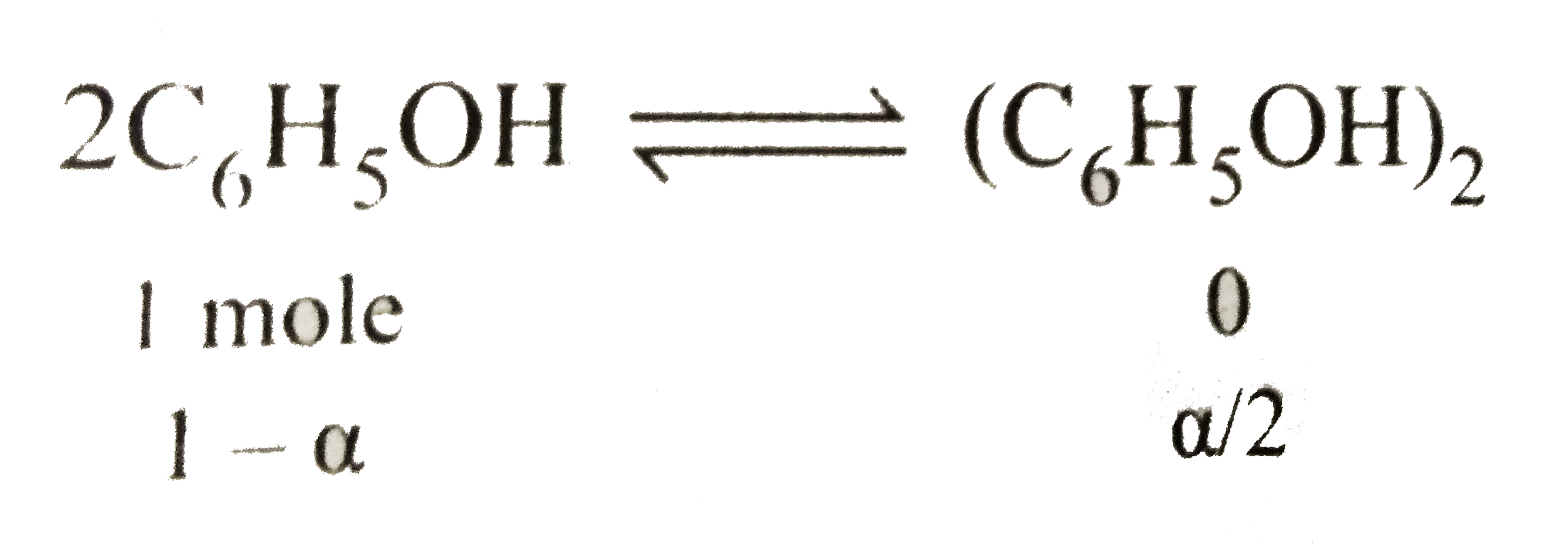
- Phenol associates in water to double molecules. The values of observed...
Text Solution
|
- Calculate the Van't Hoff factor when 0.1 mol NH(4)Cl is dissolved in 1...
Text Solution
|
- 0.5 m solution of acetic acid (Mw=60) in benzene (Mw=78) boils at 80...
Text Solution
|
- A storage battery contains a solution of H(2)SO(4) 38% by weight. At t...
Text Solution
|
- The freezing point of solution containing 0.2 g of acetic acid in 20.0...
Text Solution
|
- The degree of dissociation for PtCl(4) complex is 70%. Calculate the V...
Text Solution
|
- The degree of dissociation for K(4)[Fe(CN)(6)] is 60%. Calculate the V...
Text Solution
|
- The degree of association is 70% for the following reaction . Calculat...
Text Solution
|
- Which of the following solutions in H(2)O will show maximum depression...
Text Solution
|
- Elevation in boiling point studies of Ca(NO(3))(2) gives molar mass as...
Text Solution
|
- Phenol associates in benzene to certain extent to form a dimer. A solu...
Text Solution
|