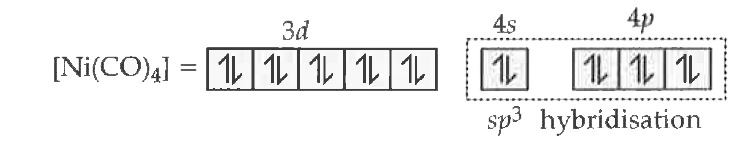
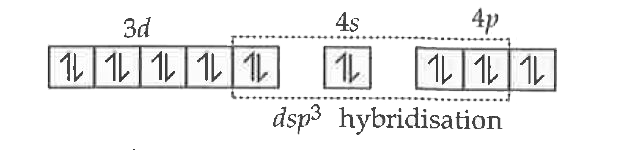
Text Solution
Verified by Experts
Topper's Solved these Questions
CO-ORDINATION COMPOUNDS
OSWAAL PUBLICATION|Exercise TOPIC-1 (CO-ORDINATION COMPOUNDS AND THEIR PROPERTIES, IUPAC NOMENCLATURE OF MONONUCLEAR CO-ORDINATION COMPOUNDS (Long Answer Type Question-I)|27 VideosCO-ORDINATION COMPOUNDS
OSWAAL PUBLICATION|Exercise TOPIC-2 (WERNER.S THEORY BONDING IN CO-ORDINATION COMPOUND , VBT, CFT AND IMPORTANCE OF CO-ORDINATION COMPOUNDS) (Very Short Answer Type Questions)|8 VideosCO-ORDINATION COMPOUNDS
OSWAAL PUBLICATION|Exercise TOPIC-2 (WERNER.S THEORY BONDING IN CO-ORDINATION COMPOUND , VBT, CFT AND IMPORTANCE OF CO-ORDINATION COMPOUNDS) (Very Answer Type Questions)|19 VideosCHEMISTY IN EVERYDAY LIFE
OSWAAL PUBLICATION|Exercise LONG ANSWER TYPE QUESTIONS-I|1 VideosD - BLOCK ELEMENTS F - BLOCK ELEMENTS
OSWAAL PUBLICATION|Exercise TOPIC - 2 (F - BLOCK ELEMENT , LANTHANOIDS AND ACTINOIDS)(LONG ANSWER TYPES QUESTIONS)|6 Videos
Similar Questions
Explore conceptually related problems
OSWAAL PUBLICATION-CO-ORDINATION COMPOUNDS-TOPIC-1 (CO-ORDINATION COMPOUNDS AND THEIR PROPERTIES, IUPAC NOMENCLATURE OF MONONUCLEAR CO-ORDINATION COMPOUNDS (Short Answer Type Questions)
- Define radius ratio. Give the limiting radius ratio value for a co-ord...
Text Solution
|
- Write the IUPAC Name of the K4 [Ni(CN)4]
Text Solution
|
- Write the IUPAC Name of the [Pt(NH3)6]Cl4
Text Solution
|
- Calculate EAN of iron in potassium ferricyanide.
Text Solution
|
- Explain linkage isomerism with example.
Text Solution
|
- Write the IUPAC name of [Co (ONO) (NH3)5] Cl2. Write the formula of a ...
Text Solution
|
- FeSO4 solution mixed with (NH4)2 SO4 solution in 1:1 molar ratio gives...
Text Solution
|
- Write all the geometrical isomers of [Pt(NH3) (Br)(C1) (py)] and how m...
Text Solution
|
- What is the co-ordination entity formed when excess of aqueous KCN is ...
Text Solution
|
- What is spectrochemical series ? Explain the difference between a weak...
Text Solution
|
- A solution of [Ni(H2 O)6]^(2+) is green but a solution of [Ni(CN)4]^(2...
Text Solution
|
- How many ions are produced from the compex,[Co(NH3)6] Cl2 is solution ...
Text Solution
|
- Write the IUPAC name of the complex [Cr(NH3)4 Cl2]^(+) . What type of...
Text Solution
|
- State reason for the following CO is stronger complexing reagent th...
Text Solution
|
- State reason for the following The molecular shape of Ni(CO)4 is no...
Text Solution
|
- Indicate the type of isomerisms exhibited by the complex [Co(NH3)5 (NO...
Text Solution
|
- Name the following co-ordination compounds according to IUPAC system o...
Text Solution
|
- Name the following co-ordination compounds according to IUPAC system o...
Text Solution
|