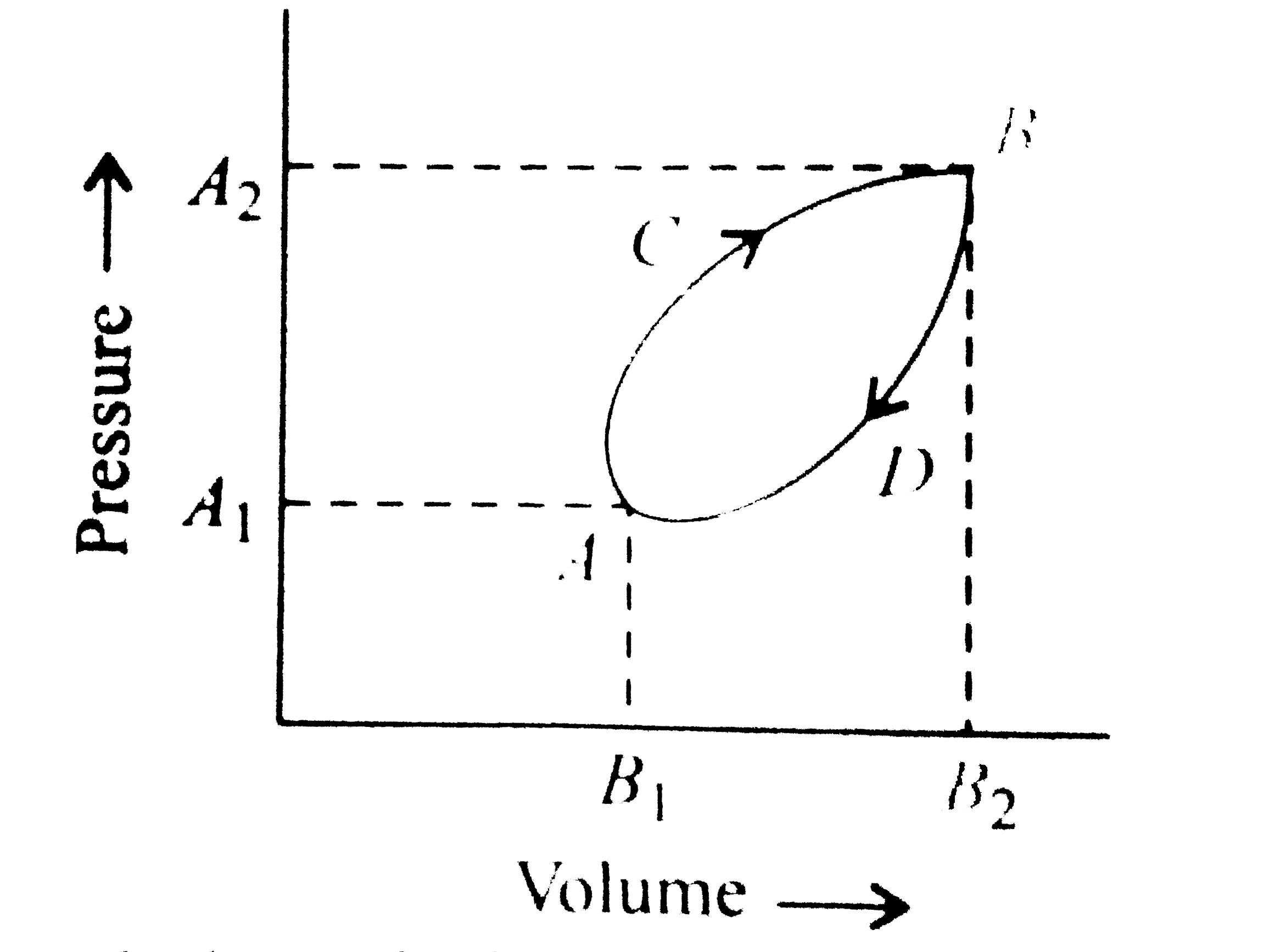
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COMPLEX NUMBERS AND QUADRATIC EQUATIONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section -D) Linked comprehension Type Questions|14 VideosCOMPLEX NUMBERS AND QUADRATIC EQUATIONS
AAKASH INSTITUTE ENGLISH|Exercise Assertion -Reason Type Questions|19 VideosCOMPLEX NUMBERS AND QUADRATIC EQUATIONS
AAKASH INSTITUTE ENGLISH|Exercise Assignment (Section -B) (objective Type Questions ( one option is correct)|78 VideosBINOMIAL THEOREM
AAKASH INSTITUTE ENGLISH|Exercise Assignment (section-J) Objective type question (Aakash Challengers Questions)|4 VideosCONIC SECTIONS
AAKASH INSTITUTE ENGLISH|Exercise SECTION - J ( Aakash Challengers Questions )|16 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE ENGLISH-COMPLEX NUMBERS AND QUADRATIC EQUATIONS-Assignment (Section -C) (objective Type Questions ( more thena one options are correct )
- If root3(-1) = -1 , -omega, -omega^(2) , then roots of the equation ...
Text Solution
|
- If z(1)=p+iq and z(2) = u = iv are complex numbers such that |z(1)|=...
Text Solution
|
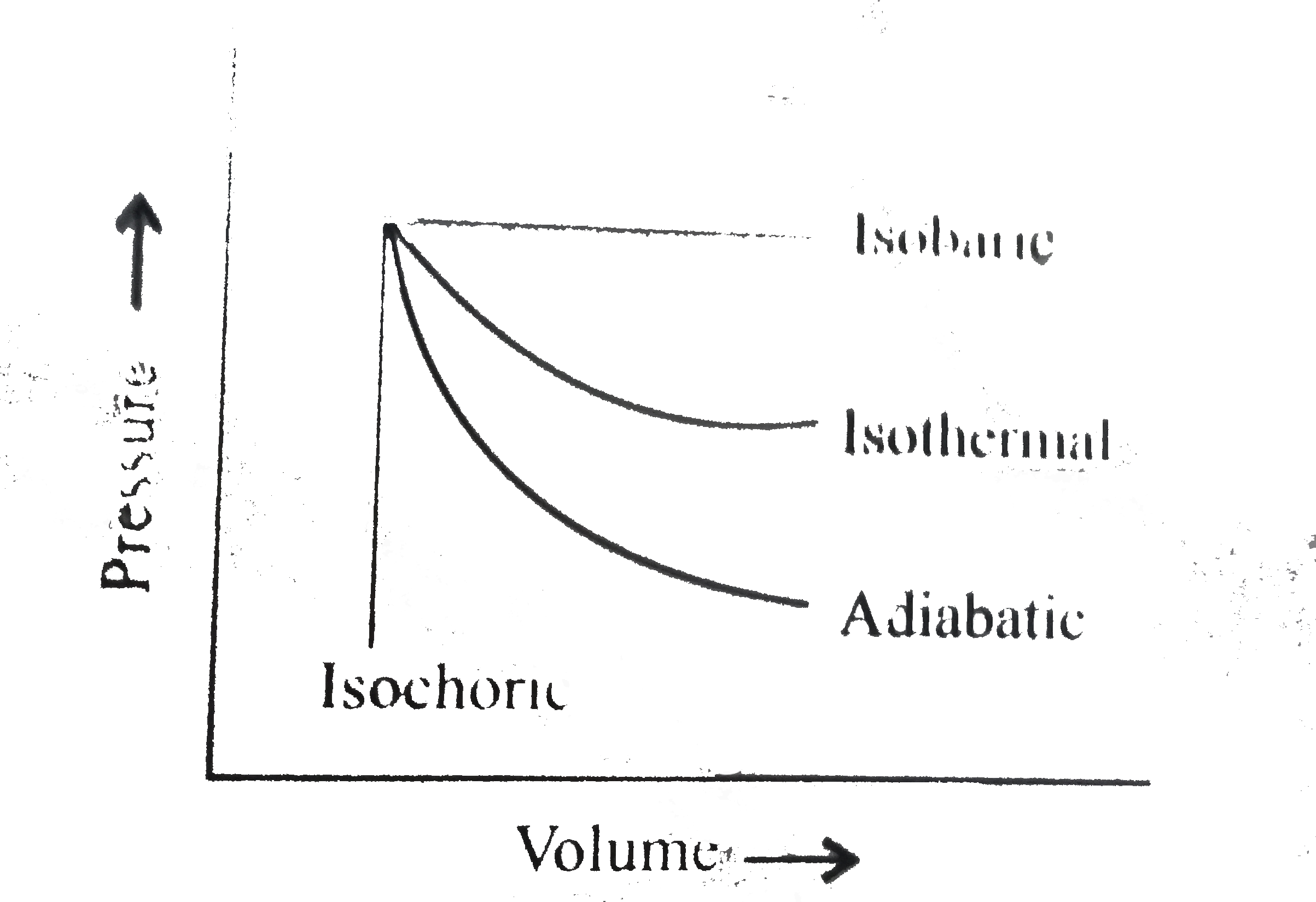
- The pressure-volume of various thermodynamic process is shown in graph...
Text Solution
|
- If z(1) ,z(2) be two complex numbers satisfying the equation |(z(1)...
Text Solution
|
- If sin alpha, cosalpha are the roots of the equation x^2 + bx + c = 0 ...
Text Solution
|
- If alpha, beta are the roots of the equation ax^(2) +2bx +c =0 and a...
Text Solution
|
- The solution set of the inequality (x+3)^(5) -(x -1)^(5) ge 244 is
Text Solution
|
- Let a,b,c be real numbers in G.P. such that a and c are positive , the...
Text Solution
|
- Let cos alpha be a root of the equation 25x^(2) +5x -12 = 0 -1 lt x...
Text Solution
|
- If the quadratic equations x^(2) +pqx +r=0 and z^(2) +prx +q=0 have a...
Text Solution
|
- The quadratic equation x^(2) - (m -3)x + m =0 has
Text Solution
|
- If both roots of the equation x^(2) -2ax+a^(2)-1=0 lie between -3 and...
Text Solution
|
- Let alpha, beta " the roots of " x^(2) -4x + A =0 and gamma, delta " ...
Text Solution
|
- For the equation x^(3/4(logx)^(2)+log(2)x-5/4)=sqrt2, which one of the...
Text Solution
|
- If f(x)=a x^2+b x+c ,g(x)=-a x^2+b x+c ,where ac!=0, then prove that f...
Text Solution
|
- Sum of the squares of all integral values of a for which the inequalit...
Text Solution
|
- If the roots of the equation 1/(x+p) + 1/(x+q) = 1/r are equal in mag...
Text Solution
|
- Find the integral values of a for which (a+2)x^2+2(a+1)x+a=0 will have...
Text Solution
|
- If (x-1)^(2) is a factor of ax^(3) +bx^(2) +c then roots of the equa...
Text Solution
|
- If b^(2)ge4ac for the equation ax^(4)+bx^(2)+c=0 then all the roots of...
Text Solution
|